Amoeba staining is the process used in laboratories for observing the structural details of free-living and intestinal amoebae. It is needed because most amoebae are transparent in fresh samples, and their internal parts cannot be seen clearly without color contrast. It is the process that helps in identifying pathogenic forms like Entamoeba histolytica from non-pathogenic amoebae by revealing the nucleus, chromatin arrangement and the presence of chromatoid bodies. In this technique the specimen is first fixed, and fixatives like Schaudinn’s solution or Polyvinyl Alcohol (PVA) are used because these prevent distortion of trophozoite stages and stop autolysis of the cells.
Temporary stains like Lugol’s iodine or lactophenol cotton blue are used in wet mounts and these are helpful for rapid examination of cysts and trophozoites. Permanent staining methods such as Wheatley’s Trichrome stain or Iron-Hematoxylin stain are used when a clear and long-lasting slide is required for species confirmation. These permanent stains give high contrast to the internal structures and the stained slide can be preserved for later observation. Some specialized stains like Calcofluor White are also used, and this stain binds to cyst walls and fluoresces under UV light. It is the method that increases the accuracy of diagnosis by showing important internal features of the amoebae.
Principle of Amoeba Staining
The principle of amoeba staining is based on creating contrast between the transparent amoebic cell and the background so that its internal structures can be clearly observed. It is the process that alters the optical properties of the organism, and this helps in identifying diagnostic structures like the nucleus, karyosome and the different locomotory parts.
Amoebae are mostly translucent and mixed with debris in the sample, therefore staining is required to highlight the internal parts which otherwise is not visible. The staining always begins after fixation, and fixatives like methanol, Schaudinn’s solution or PVA is used to stop autolysis and to preserve the protoplasm so that the original shape is maintained.
After fixation, the interaction between the dye and the cellular components determines the contrast. In permanent staining like Iron Hematoxylin and Wheatley’s Trichrome, there is specific chemical affinity between the dye and the nuclear material. Iron Hematoxylin forms a lake complex between the oxidized dye (hematein) and the metal mordant, and this behaves like a basic dye that binds to the acidic nuclear substances giving a dark stain to chromatin and chromatoid bodies while the cytoplasm remain lighter.
In Trichrome staining, different dyes are used and these give a blue-green cytoplasm and red to purple nuclear parts which makes the structures clearly visible. Temporary stains like Lugol’s iodine or lactophenol cotton blue work by simple penetration into the cell and produce quick color contrast for observing motility and external morphology. It is the technique that depends on dye affinity and fixation to show differential staining of the amoebic structures.
Requirements for Amoeba Staining
- Fixation Requirements
- Fixation is the process that help in stopping autolysis and putrefaction of the cells. It is required to preserve cytoplasm and nuclear structures.
- Trophozoites are fragile and disintegrate fast in fresh stool, so immediate fixation is needed to maintain the karyosome and peripheral chromatin.
- Schaudinn’s solution is used for permanent smears and it preserve fine morphological details.
- Polyvinyl alcohol (PVA) is used when permanent stained smears is required, as it binds the specimen to the slide.
- Formalin can preserve cysts but it is not suitable for trophozoites for permanent staining.
- Fixation solutions are kept near 37°C to prevent thermal shock which cause retraction or rounding of the cells.
- Requirements for Permanent Staining Techniques
- Permanent stains help in making a stable record of amoeba morphology. Wheatley’s Trichrome Stain
- Iodine-alcohol is required to remove mercury crystals when mercury fixatives are used.
- A differentiation step is done using acidified alcohol for separating cellular components.
- Cytoplasm stain blue-green while nuclei and inclusions stain red or purple. Iron–Hematoxylin Stain
- Hematoxylin must be ripened before staining.
- A mordant like iron alum is used to bind the dye to the tissue.
- The method is regressive, so overstaining and careful differentiation is required.
- Rinse water must be neutral because alkaline water interfere in differentiation and acidic water destain too fast.
- Requirements for Temporary and Rapid Staining
- Temporary stains are used in rapid identification and free-living amoebae examination.
- Lugol’s iodine is a simple iodine-potassium iodide solution giving quick contrast for nuclei in wet mounts.
- Lactophenol Cotton Blue stains double walls of Acanthamoeba cysts dark blue without complex fixation.
- Calcofluor white require a fluorescence microscope with UV emission because the stain binds to cellulose and chitin, forming bright blue-white halos.
- Quality Control and Troubleshooting Requirements
- Water quality is important, especially for hematoxylin stains where distilled or deionized water is needed for critical rinsing.
- Permanent slides are dehydrated in graded ethanol series and then cleared in xylene to avoid cloudiness.
- A known positive control slide is used in every staining run to check the efficiency of reagents and procedure.
Step by Step Procedure of Amoeba Staining
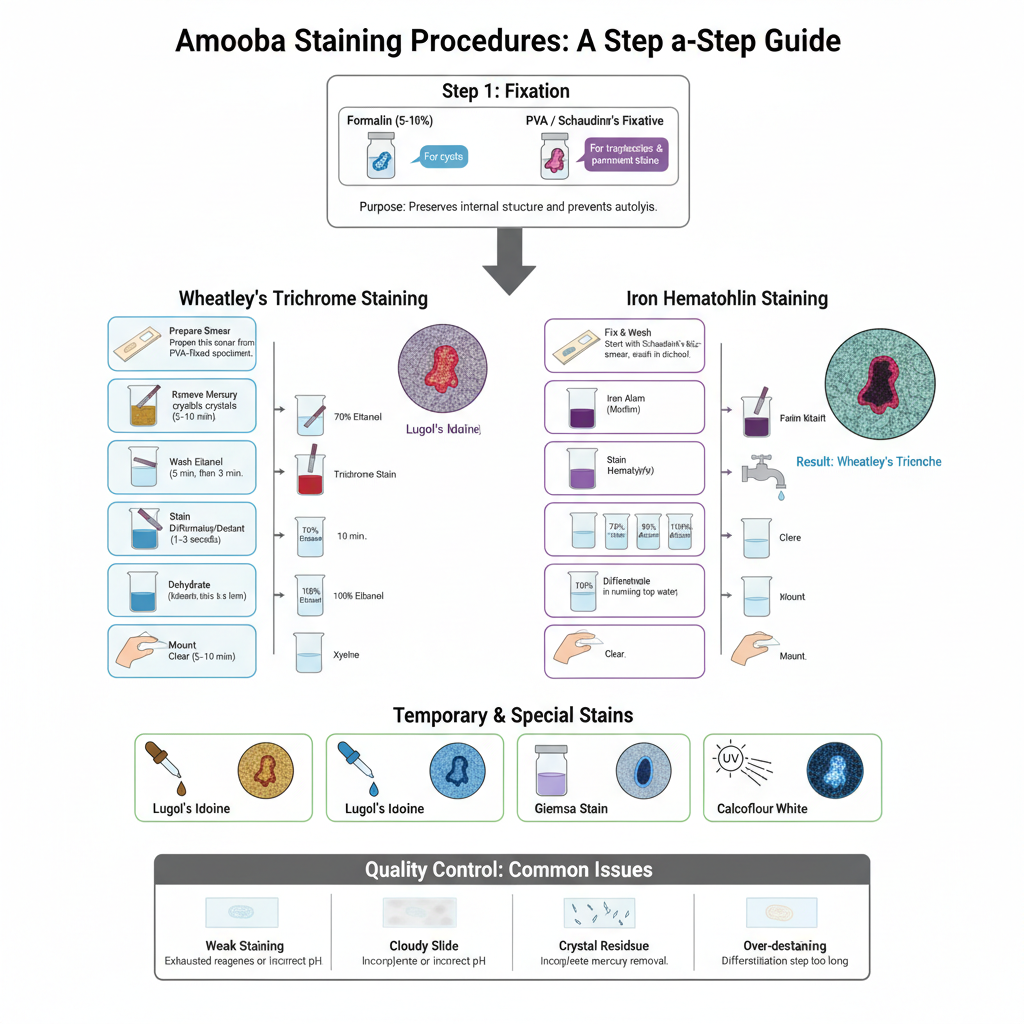
- Fixation
- It is the first step and the specimen is fixed to prevent autolysis and to preserve the internal structure.
- In intestinal amoebae two vial system is used.
- Formalin (5% or 10%) is used for preservation of cysts and also help in concentration process.
- PVA or Schaudinn’s fixative is required for permanent staining. PVA keep the specimen attached on slide during staining while Schaudinn’s solution provide better morphological detail.
- In free living amoebae fixatives like Nissenbaum’s or Carnoy’s solution is used especially when fluorescent stain is done.
- Wheatley’s Trichrome Staining
- A thin smear is prepared from PVA fixed specimen on slide and it is allowed for drying.
- If mercury fixative is used then slide is placed in iodine alcohol (70% ethanol + iodine) for 5–10 minutes for removal of mercury crystals.
- Slide is transferred in 70% ethanol for 5 minutes and again in fresh 70% ethanol for 3 minutes.
- In this step the slide is placed inside Trichrome stain for 10 minutes.
- Destaining is done by dipping in 90% ethanol + acetic acid for 1–3 seconds. This is referred to as differentiation step.
- Slide is rinsed quickly in 100% ethanol and then in two changes of 100% ethanol for 3 minutes each for dehydration.
- Clearing is done in xylene for 5–10 minutes.
- Mounting is done with coverslip using mounting media.
- Result – Cytoplasm appear blue green and nuclei with chromatoid bodies appear red or pinkish red.
- Iron Hematoxylin Staining (Heidenhain Method)
- Smear is fixed in Schaudinn’s solution and washed in 70% alcohol. Mercury removal is done by iodine alcohol if required.
- In this step the slide is placed in mordant (iron alum) for 4–5 minutes.
- Slide is then stained in hematoxylin for 4–5 minutes and slide become dark.
- Differentiation is carried out in running tap water until cytoplasm become clear while nucleus retain the colour.
- Slide is passed through 70%, 95% and 100% alcohol for dehydration and then cleared in xylene.
- Result – Nuclear chromatin appear dark purple to black while cytoplasm appear gray or violet.
- Temporary and Special Stains
- Lugol’s Iodine (Wet Mount)
- A drop of iodine is added to fresh specimen on slide and covered with coverslip.
- Cytoplasm become yellow brown and nucleus is clearly visible.
- Lactophenol Cotton Blue
- Sediment is mixed with stain and coverslip is applied.
- Cyst wall stain dark blue and internal structures become visible.
- Giemsa Stain
- Smear is fixed in methanol for 5 minutes and placed in dilute Giemsa for 20 minutes then washed.
- Trophozoite appear blue and nucleus appear darker.
- Calcofluor White Stain
- Smear is fixed in methanol and stained with 0.01% Calcofluor for 1 minute then observed under UV.
- Cyst wall fluoresce blue white which help in quick detection.
- Lugol’s Iodine (Wet Mount)
- Quality Control Points
- Weak staining is seen when reagent become exhausted or pH of rinse water is not correct.
- Cloudy slide occur when dehydration is incomplete and water remain during xylene step.
- Crystal residue appear due to incomplete mercury removal.
- Over destaining remove all colour so timing is important in differentiation step.
Result of Amoeba Staining
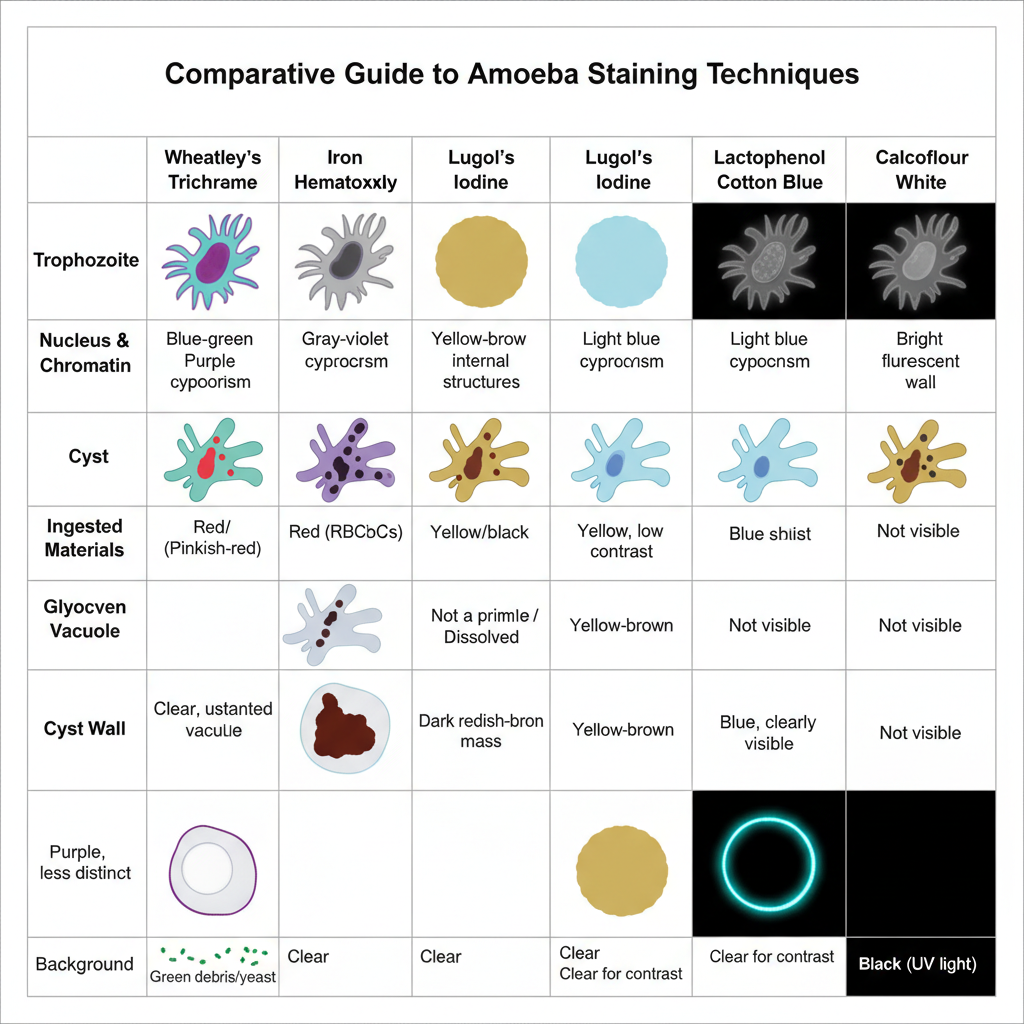
- Wheatley’s Trichrome Stain
- Cytoplasm of trophozoites appear blue green and sometimes a slight purple shade is seen.
- Cysts usually appear more toward purple colour.
- It is the nucleus, chromatin and chromatoid bodies that stain red or pinkish red which help in identification.
- Ingested RBCs also appear red.
- Background debris and yeast cells stain green.
- Glycogen become dissolved during the process so it appear as clear unstained vacuole inside the cell.
- Iron Hematoxylin Stain
- Cytoplasm of trophozoites and cysts appear violet or gray.
- Nuclear chromatin and chromatoid bodies stain dark purple to black giving sharp contrast.
- Ingested bacteria and erythrocytes also show dark staining.
- In free living amoebae (Acanthamoeba) both outer and inner cyst wall appear dark gray under this stain.
- The nucleus inside cyst sometimes is not clearly visible.
- Lugol’s Iodine (Temporary Stain)
- Internal structures appear yellow after iodine stain.
- In some species like Iodamoeba buetschlii a large glycogen vacuole appear as dark reddish brown mass.
- It is useful for quick observation but staining is not permanent.
- Lactophenol Cotton Blue
- Cytoplasm and cell wall appear blue in organisms like Naegleria and Acanthamoeba.
- It provide quick contrast with the background.
- Cyst wall is seen more clearly in this stain.
- Calcofluor White (Fluorescent Stain)
- Cyst wall containing cellulose fluoresce bright blue white or turquoise under UV light.
- It help in easy detection especially in corneal scraping.
- Internal structures is not well visible in this stain.
Uses of Amoeba Staining
- It is used for the definitive diagnosis of amoebae because stained smears show the nuclear structures clearly which is required to differentiate pathogenic species from non-pathogenic ones. It is the process where the karyosome position and the peripheral chromatin can be seen and it helps in separating Entamoeba histolytica from E. coli or E. hartmanni.
- It is used for observing intracellular morphological details of the trophozoites and cysts. Nuclear membrane, chromatin pattern, and karyosome becomes distinct in permanent stains. Cytoplasmic inclusions like chromatoid bodies or glycogen vacuoles is also seen in this step which help in identification of different species.
- It is used to visualize locomotor structures. Acanthopodia in Acanthamoeba or lobopodia in Naegleria is observed after staining because these structures is not clearly visible in unstained cells.
- It is used for rapid screening of free-living amoebae in clinical samples. In keratitis diagnosis Calcofluor White stain is used and it binds with cyst wall producing a bluish fluorescence which help in quick detection of cysts when the number is low.
- It is used for environmental surveillance. Temporary stains like Lugol’s iodine and Lactophenol Cotton Blue give contrast to water samples and it helps in quick screening of large number of specimens without preparing permanent slides.
- It is used to assess viability of living amoebae. Supravital stains such as Janus Green B stain mitochondria and Neutral Red accumulates in vacuoles, so it helps in studying metabolic activity and phagocytic behaviour of the cell.
- It is used to make permanent archival records. Permanent stained smears can be stored for a long time, allowing re-examination, teaching use, and reference study. It is important in quality control programs as the slides remain unchanged and can be checked again when diagnosis is uncertain.
Advantages of Amoeba Staining
- It is the main method for observing internal morphological details because stained preparations show the nucleus, karyosome and peripheral chromatin clearly. These are important for differentiating E. histolytica from the non-pathogenic species. Cytoplasmic inclusions like chromatoid bodies and glycogen vacuoles is also seen after staining which help in species identification.
- It is the process that preserves fragile trophozoites. Fresh trophozoites undergo rapid lysis and autolysis, but fixation stops this self-digestion and keeps the protoplasm stable. Structural integrity of cytoplasm and nucleus is maintained when fixatives like PVA or Schaudinn’s solution is used.
- It is used for preparing permanent archival slides. Permanent stained smears remain stable for long periods and can be re-checked for confirmation of diagnosis. These slides is useful for teaching, reference work, and quality control because wet mounts degrade quickly and cannot be stored.
- It gives method-specific diagnostic advantages. Temporary stains provide quick screening for free-living amoebae, while Trichrome staining is simple and useful for routine examination. Iron-hematoxylin gives sharp high-resolution detail which is needed for difficult taxonomy. Fluorescent stains like Calcofluor White offer rapid detection of cyst walls by producing bluish-white fluorescence.
- It is the process that helps in detecting co-infections. Modified Acid-Fast stain is used for coccidian oocysts which may not be visible in routine stains. Chromotrope staining helps in identifying microsporidia spores by giving contrast from fecal debris.
Limitations of Amoeba Staining
- It is limited because no single fixative preserves all stages equally well. Formalin keeps cysts and helminth eggs intact but trophozoites is poorly preserved and often become unrecognizable. PVA or Schaudinn’s solution preserve trophozoites well but cannot be used for concentration procedures, so laboratories depend on two separate vials which increases work and cost.
- It is affected by fixation artifacts. When the fixative is too concentrated or too dilute the cells can shrink or swell which can disturb the internal structure and may cause confusion during identification.
- It is technically demanding when permanent stains are used. Iron hematoxylin staining takes a long time and the differentiation step is very sensitive, and under-differentiation produces dark slides while over-differentiation removes important details. Tap water with alkaline pH also reduces the staining capacity and the nuclear structures is not clearly visible.
- It is restricted by artifacts in Trichrome staining. Glycogen is dissolved during the staining process, leaving empty spaces which sometimes interfere with identifying species. When mercury fixatives are used, an iodine-alcohol step is needed or mercuric chloride crystals remain on the slide and these crystals obscure the view.
- It has limitations when temporary stains are used. Wet mounts dry out quickly and cannot be stored. Iodine may distort the organism. Lactophenol Cotton Blue stains cysts well but trophozoites appear diffuse making recognition difficult. Gram stain and Acid-fast stain is not suitable for amoebae as they do not show the nuclear pattern required for species differentiation.
- It involves safety issues due to the chemicals used. Mercuric chloride in classical fixatives is toxic and makes waste disposal difficult. Other reagents may also irritate skin or respiratory tract and need careful handling.
- It is prone to staining artifacts. Precipitates from old solutions, debris in water baths, or heat applied during drying can cause bubbling in the nucleus. Intake of barium, mineral oil or antidiarrheal drugs by the patient before sample collection also produce unsatisfactory smears for several weeks.
- Alpha-Tec Systems. (n.d.). PVA fixatives (Zn) formalin (10% and 5%) buffered fixatives [Instructions for Use]. Calibre Scientific.
- Centers for Disease Control and Prevention. (2016, May 3). DPDx – Diagnostic procedures – Stool specimens – Specimen collection. https://www.cdc.gov/dpdx/diagnosticprocedures/stool/specimencoll.html
- Centers for Disease Control and Prevention. (2016, May 3). DPDx – Diagnostic procedures – Stool specimens – Staining procedures. https://www.cdc.gov/dpdx/diagnosticprocedures/stool/staining.html
- Centers for Disease Control and Prevention. (2019, October 29). DPDx – Intestinal (non-pathogenic) amebae. https://www.cdc.gov/dpdx/intestinalamebae/index.html
- da Silva, M. B., Aguiar, C. d. S., Rocha, C. V. S., Santos, S. B., dos Anjos, M. S., Silva, R. S. V., Nishiyama, P. B., Fraga, R. E., Rocha, M. A., & Oliveira, P. P. (2020). Glyceryn-jelly as mounting medium for permanent slides for protists and rotifers: A proposal for didactic purposes. Biotemas, 33(4), 1–6. https://doi.org/10.5007/2175-7925.2020.e75404
- Eldeek, H. E. M., Attia, R. A. H., Nageeb, M. M., & Sakla, A. A. (2019). Comparative evaluation of multiple staining techniques for identification of different developmental stages of Acanthamoeba and Naegleria. Journal of the Egyptian Society of Parasitology, 49(2), 409–422.
- El-Sayed, N. M., & Hikal, W. M. (2015). Several staining techniques to enhance the visibility of Acanthamoeba cysts. Parasitology Research, 114(3), 823–830. https://doi.org/10.1007/s00436-014-4190-4
- Ethos Biosciences. (n.d.). Lab technician’s guide to troubleshooting: 20 common issues with biological stains [E-book].
- Ferreira, C. S. (2003). Staining of intestinal protozoa with Heidenhain’s iron hematoxylin. Revista do Instituto de Medicina Tropical de São Paulo, 45(1), 43–44. https://doi.org/10.1590/S0036-46652003000100009
- Fisher Diagnostics. (n.d.). Protocol trichrome stain [Instructions for Use]. Thermo Fisher Scientific.
- Grafe, M., Pitzen, V., Meyer, I., & Gräf, R. (2024). Superresolution expansion microscopy in Dictyostelium amoebae. Methods in Molecular Biology, 2814, 29–44. https://doi.org/10.1007/978-1-0716-3894-1_2
- Greider, M. H., Kostir, W. J., & Frajola, W. J. (2007). Electron microscopy of Amoeba proteus. Journal of Eukaryotic Microbiology, 5(2), 139–146. https://doi.org/10.1111/j.1550-7408.1958.tb02541.x
- Maciver Lab. (2003, February 3). Fixing and staining amoebae. University of Edinburgh. https://maciverlab.bms.ed.ac.uk/Staining_amoebae.htm
- Merck KGaA. (2017). Microscopy Neutral red (C.I. 50040) – for microscopy Certistain® [Instructions for Use].
- Mokobi, F. (2022, May 4). Iron-hematoxylin staining. Microbe Notes. https://microbenotes.com/iron-hematoxylin-staining/
- National Society for Histotechnology. (2024, January 26). Troubleshooting fixation in histology pt 2: Advanced troubleshooting and pro tips. https://www.nsh.org/blogs/ashley-stewart/2024/01/26/troubleshooting-fixation-in-histology-pt-2
- Remel. (2012). PVA modified fixative [Instructions for Use]. Thermo Fisher Scientific.
- Sampias, C. (n.d.). H&E basics part 4: Troubleshooting H&E. Leica Biosystems.
- Wu, A. (2016, March 28). Problem with cell deteaced/morphological changed after fixation with 4%PFA, How should I optimize the protocol? [Online forum post]. ResearchGate.
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.