What is Nanoparticle-Assisted PCR (nanoPCR)?
- Nanoparticle-Assisted PCR (nanoPCR) was developed as an Modified form of the Polymerase Chain Reaction where Nanoparticles are used for Enhancing efficiency.
- In this method, the amplification of DNA molecules is improved by addition of nanoparticles like gold (Au), silver (Ag), or other metallic/ non-metallic particles.
- The nanoparticles are believed to change the thermal conductivity of reaction mixture, which results into more uniform heat distribution inside the PCR tube.
- By this process, denaturation, annealing and extension stages of DNA replication are stabilized and speeded, even when standard PCR conditions may not perform well.
- The nanoPCR has been used for higher sensitivity detection of viral, bacterial and genetic materials, which makes the method very useful in diagnostic laboratories.
- The specificity of primers is improved because unwanted binding/ mispriming reactions are reduced by influence of nanoparticles on DNA hybridization kinetics.
- In the presence of nanoparticles, smaller quantities of template DNA were amplified successfully, which means the detection limit is lowered compared to Conventional PCR.
- The gold nanoparticles especially are reported to interact with DNA molecules and polymerases, and because of this interaction amplification accuracy is enhanced (PNAS paper Sanchez, Pierce et al. 2004).
- In several research reports, amplification efficiency was increased at lower cycle numbers, so the total time of PCR reaction is shortened which is highly advantageous.
- The reaction mixture in nanoPCR is often prepared with nanoparticles suspended in buffer solution, although exact concentration and size of nanoparticles may vary among studies.
- For molecular diagnostic application, nanoPCR was considered more reliable than classical PCR, because weaker signals of DNA fragments are more easily detected.
- From biological viewpoint, the nanoparticles act as thermal regulators, while at the same time their surface properties can interact with nucleic acids.
- The nanoPCR has been widely explored for detection of RNA viruses after reverse transcription step, which is very important in emerging viral infections.
- It must be remembered that optimization of nanoparticle concentration is necessary because excessive amounts may inhibit DNA polymerase activity.
- The advantages of nanoPCR are therefore associated with enhanced sensitivity, improved specificity, shortened reaction time, and expanded application spectrum in diagnostics and research.
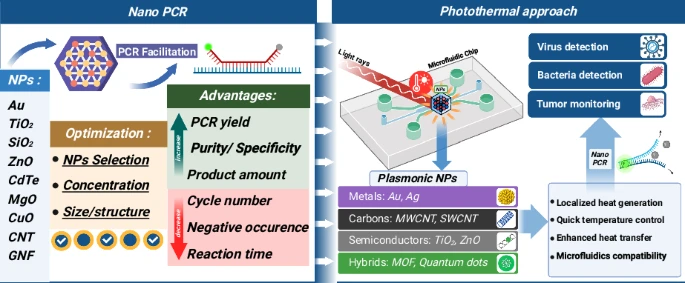
Working Principle of Nanoparticle-Assisted PCR (nanoPCR)
The nanoPCR method is introduced with nanoparticles (NPs) being added into a conventional PCR mixture which contains template DNA, primers, DNA polymerase, dNTPs, and buffer.
With optimal NP size (often <100 nm) and concentration being selected, thermal conductivity is enhanced by NPs which accelerates heating/cooling phases, thereby reducing denaturation‐annealing‐extension transition times.
Surface interactions are involved: NPs interact with primers, template, polymerase, via van der Waals, electrostatic or hydrophobic forces, which modulate component binding/unbinding dynamics.
Primer adsorption is effected: mismatched primers may be preferentially adsorbed by NPs, while correctly matched primer-template duplexes less so; this improves specificity of annealing.
Polymerase activity is modulated: some polymerase molecules are adsorbed or sequestered by NP surfaces, which reduces non-specific extension and lowers off-target amplification.
Product adsorption / dissociation assistance is provided: PCR products (amplicons) may interact with NPs; during denaturation their dissociation is aided which supports cleaner cycling and reduced artifacts.
Electrostatic and charge effects are utilized: NPs with positive surface charge attract negatively charged DNA (templates/primers), influencing hybridization dynamics and reducing primer-dimer or secondary structure formation.4
Some NPs operate via photothermal / alternative heating mechanisms: light‐absorbing NPs convert photon energy into heat (plasmonic localised heating, non-radiative relaxation etc.), which supports faster or more uniform thermal cycling.
Through the combined effects of improved thermal transfer, modulated polymerase / primer interactions, surface / charge effects, and possibly photothermal action, the following outcomes are produced: increased sensitivity (lower detection limits), enhanced specificity (less non-specific amplification), shorter reaction times, and sometimes more reliable amplification of difficult templates (GC-rich, long amplicons).
Constraints must be considered: improper NP concentration or size can inhibit polymerase or interfere with amplification; overadsorption of primers or polymerase can reduce yield; thermal inhomogeneity may also occur if NPs aggregate or their properties are not uniform.123
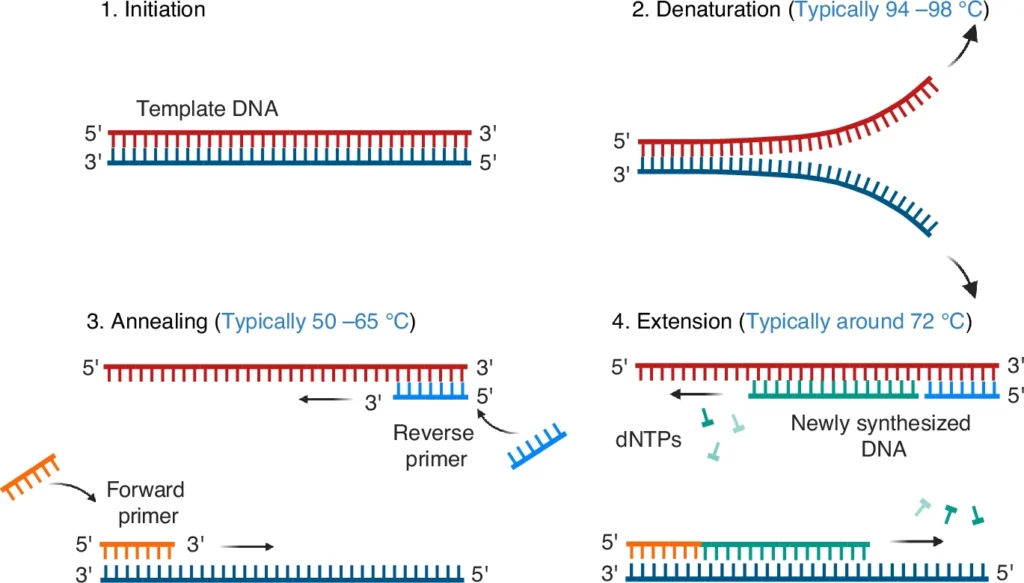
Steps / Protocols of Nanoparticle-Assisted PCR (nanoPCR)
- The Preparation of PCR Mixture is first carried out, where the DNA template, primers, deoxynucleotide triphosphates (dNTPs), and the buffer solution are taken.
- To this mixture, the DNA polymerase enzyme is added which will perform extension.
- A required concentration of nanoparticles (AuNPs, Fe₃O₄ NPs, or other functionalized particles) is then included into the same reaction mixture.
- Optimization of nanoparticle concentration is always done before full reaction because excessive NPs are inhibitory, but insufficient ones are ineffective, and therefore correct balance is maintained.
- From the mixture, aliquots are dispensed into PCR reaction tubes which already have been sterilized, and contamination should be avoided as far as possible.
- The Initial Denaturation step is performed at high temperature (94–95 °C for about 3–5 minutes), during which the double stranded DNA is separated into single strands, and nanoparticles contribute to faster and more uniform thermal transfer.
- Annealing stage is carried out at a lower temperature (50–65 °C, depending on primer Tm) in which primers hybridize with the complementary regions of the template.
- By nanoparticles, mismatched primers are suppressed through surface adsorption, while correct primers remain hybridized.
- Extension step is performed at optimum temperature for DNA polymerase (commonly 72 °C), and the enzyme incorporates nucleotides to form new complementary strand.
- Polymerase activity is modulated by NPs because some molecules are transiently bound, which prevents non-specific synthesis.
- The PCR cycle is repeated typically 25–40 times, each including denaturation / annealing / extension, and during every cycle nanoparticles assist in heat transfer and in fidelity of reaction.
- After completion, a Final Extension is carried out at 72 °C for 5–10 minutes, ensuring that all DNA fragments are fully elongated.
- Amplification products are then cooled and stored at 4 °C, and these products can be later subjected to Gel electrophoresis, sequencing, or diagnostic analysis.
- Quality Control is recommended at every step, because nanoparticle aggregation, uneven distribution or excess adsorption on primers can cause experimental variation.
- In the optional protocols, functionalized nanoparticles (with surface groups, ligands, antibodies) are employed, and in such cases additional preparation steps are included for surface modification before mixing with PCR reagents.
- The Overall Protocol is almost similar to conventional PCR, but only with addition of nanoparticles and with slight alterations in cycle timing, and sometimes in annealing temperature.
Applications of Nanoparticle-Assisted PCR (nanoPCR)
- The Detection of Viral Pathogens is enabled, and sensitivity is improved over conventional PCR which allows earlier virus identification (for example bovine respiratory syncytial virus detection).
- In the Diagnosis of Animal Diseases, nanoPCR has been employed for simultaneous detection / differential diagnosis of co-infections (virus / bacteria) in pets or livestock, which reduces misdiagnosis, and speeds up response.
- For Surveillance / Field Monitoring, pathogens in clinical or field samples are detected more reliably because low copy number nucleic acid is amplified with high specificity, thus false negatives reduced.
- The Distinguishing Of Closely Related Strains (or wild-type vs vaccine strains) is performed, because nanoPCR often can discriminate small genetic differences, or allelic variation.
- In Veterinary Virology, nanoPCR is applied widely for detecting animal viruses (for example FCV, FHV-I in cats; porcine viruses etc.), because its high sensitivity helps in early detection in animal populations.
- The Food Safety / Environmental Testing is supported: pathogens or microbial contaminants in food or in environmental samples are identified more accurately when nanoPCR is used, due to its enhanced detection limits.
- Use in Multiplex / Dual Detection Assays is done: more than one pathogen can be simultaneously identified in same reaction using nanoPCR (dual detection), saving time and resources.
- In Clinical Diagnostics of Human Pathogens, nanoPCR is being explored for detecting human viruses / bacteria at early/low load, especially in samples where inhibitors / low template concentration are issues.
- Research / Mechanistic Studies are advanced: nanoPCR is used to understand PCR inhibition by substances, thermal transfer improvements, nanoparticle-polymerase / primer interactions etc., which contributes to improved PCR designs.
- In Vaccine Monitoring / Quality Control, nanoPCR has been used to distinguish between vaccine strains and wild-type infections, which is important in epidemiological tracking and vaccine efficacy studies.56
Advantages of Nanoparticle-Assisted PCR (nanoPCR)
- The Sensitivity of amplification is increased, because target nucleic acid at low copy number is amplified more efficiently when nanoparticles (NPs) are included.
- Specificity is enhanced by suppression of non-specific amplification, through primer / polymerase interactions with NP surfaces, which reduce mis-priming and primer-dimer formation.
- The Reaction time is reduced, since thermal conductivity improvements by NPs allow faster heating/cooling cycles and sometimes fewer cycles are needed.
- Broadening of Annealing Temperature Range is effected, enabling more flexible primer design or more forgiving conditions for template/primer mismatches.
- Yield of PCR product is improved, because more efficient extension / amplification is allowed by improved heat transfer and reduced inhibition.
- Low Template/Inhibitor Tolerance is increased, because NPs can mitigate effects of inhibitors or low template amounts, making detection more reliable in complex samples.
- Resource / Energy Savings are possible, because cycle times are shortened / fewer cycles or lower temperature annealing required, thereby less energy / reagents consumed.
- Flexibility in NP Types / Functionalization is present, because different nanomaterials (gold, metal oxides, quantum dots, carbon-based) can be used, and surface modifications tailored, which allows adaptation to specific templates or sample types
- Improvement in Reproducibility is achieved, since when proper NP type/concentration is optimized, more consistent amplification (less variability) is observed.
- Compatibility with Downstream Applications is maintained, because nanoPCR products are usable in sequencing, diagnostic assays, or gel electrophoresis similarly to conventional PCR, while offering superior properties.
Limitations of Nanoparticle-Assisted PCR (nanoPCR)
- The Optimization requirements are heavy, because nanoparticle size, concentration, surface chemistry, primer design, annealing temperature etc must be tuned individually, which increases time before workable protocol is obtained.
- Inconsistent Results may be produced, when NP dispersion is poor, aggregation occurs, or when surfaces are not uniformly functionalized, which causes variability between replicates or between labs.
- Enzyme Inhibition is possible, because certain nanoparticles bind or adsorb polymerase or template, reducing enzyme activity or template availability, which lowers yield or can abolish amplification entirely.
- Reduced Fidelity is sometimes noted, when error rates are increased; NP-induced DNA damage or disturbed polymerase functionality may lead to misincorporation, which is problematic for applications like sequencing.
- Cost / Material Complexity is increased, because well-defined nanoparticles (of correct size, stable colloidal behaviour, functionalization) are more expensive, and additional reagents / quality control steps are needed. (Less commonly discussed in literature but implied by need for NP quality).
- Potential for Non-Specific or Background Amplification remains, when wrong NP types or over-use is made; adsorption of primers / templates non-selectively may lead to primer-dimer or spurious products.
- Interference with Reaction Components (dyes, dyes quenching, fluorescent reporters etc) is possible, since some NPs absorb/fluoresce or scatter light, or interact with intercalating dyes / probes used for detection, causing signal distortion or loss.
- Sample-Type Dependence is strong, because in complex sample matrices (soil, faeces, blood etc) inhibitors / contaminants may interact with NPs in unpredictable ways, which may reduce the benefit or even worsen PCR performance.
- Scalability Issues are present, because in high-throughput or diagnostic lab settings consistent NP quality, reproducible batches, and standardization can be hard. yielding batch-to-batch variation.
- Long-Term Stability / Storage Problems may be encountered, when nanoparticles degrade, oxidize, aggregate over time, or when coatings / functionalization is lost, which leads to reduced enhancement or inconsistent effects.
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.