- The Polymerase Chain Reaction (PCR) is a laboratory technique that is used to amplify (make many copies of) a specific segment of DNA.
- A DNA template is required, from which the target DNA region is defined, so that primers (short single–stranded DNA oligonucleotides) can bind flanking the region
- Denaturation is performed by heating the DNA to separate its two strands.
- Annealing is done when temperature is lowered, so that primers hybridize to complementary sequences on each single‐stranded template.
- Extension is carried out by a thermostable DNA polymerase enzyme which adds nucleotides to the 3′ ends of primers, thereby synthesizing new DNA strands.
- Thermal cycling (repeated cycles of denaturation, annealing, extension) is carried out many times (e.g., 20-40 cycles) to produce exponential amplification of the target DNA segment.
- Taq polymerase (derived from Thermus aquaticus) is often used because of its ability to survive the high denaturation temperatures.
- The small amount of starting DNA is converted into millions/billions of copies, so that enough material becomes available for subsequent study (sequencing, diagnosis, cloning, etc.)
- The PCR process is sensitive which means contamination can lead to amplification of non‐target DNA, giving false positives.
- Specificity is dependent on proper primer design, correct annealing temperature, and integrity of polymerase & reagents.
Types of PCR
Here is the list of Types of PCR based on different categories;
- Standard PCR Types
- Conventional PCR – Basic endpoint PCR amplification
- High-fidelity PCR – Enhanced accuracy with proofreading polymerases
- Hot-start PCR – Prevents non-specific amplification at room temperature
- Fast-cycling PCR – Rapid amplification with shortened cycle times
- Touch down (TD) PCR – Gradually decreasing annealing temperatures
- Real-Time PCR Types
- Real-Time PCR (qPCR) – Quantitative PCR with real-time monitoring
- Digital PCR (dPCR) – Absolute quantification through sample partitioning
- High-Resolution Melt (HRM) PCR – Real-time PCR with melting curve analysis
- RNA-Based PCR Types
- Reverse-Transcriptase PCR (RT-PCR) – RNA to cDNA conversion and amplification
- RT-qPCR – Real-time quantitative reverse transcription PCR
- RNase H-dependent PCR (rhPCR) – RNA-specific amplification method
- Multiplex and Complex PCR Types
- Multiplex PCR – Simultaneous amplification of multiple targets
- Nested PCR – Two-round amplification for enhanced specificity
- Long-range PCR – Amplification of large DNA fragments (>5kb)
- Assembly PCR – Synthetic gene construction method
- Overlap extension PCR – Site-directed mutagenesis and gene fusion
- Genetic Analysis PCR Types
- Allele-specific PCR – Discriminates between allelic variants
- AFLP PCR – Amplified fragment length polymorphism analysis
- VNTR PCR – Variable number tandem repeat analysis
- ISSR PCR – Inter-simple sequence repeat amplification
- Methylation-specific PCR (MSP) – Detects DNA methylation patterns
- SSP-PCR – Single specific primer PCR for HLA typing
- Specialized Application PCR Types
- Colony PCR – Direct amplification from bacterial colonies
- Single cell PCR – Amplification from individual cells
- In situ PCR – Amplification within tissue sections
- Solid phase PCR – Surface-bound amplification
- COLD PCR – Preferential amplification of minority variants
- Advanced PCR Types
- Asymmetric PCR – Unequal primer concentrations for single-strand products
- LATE PCR – Linear after exponential amplification
- Inverse PCR – Amplification of unknown flanking sequences
- TAIL-PCR – Thermal asymmetric interlaced PCR for genome walking
- Ligation-mediated PCR – Unknown sequence amplification with adaptors
- Repetitive Element PCR Types
- Alu PCR – Amplification between Alu repetitive elements
- Repetitive sequence-based PCR – Uses repetitive DNA as primer sites
- Emerging PCR Types
- Nanoparticle-Assisted PCR (nanoPCR) – Enhanced efficiency with nanoparticles
- Miniprimer PCR – Ultra-short primer amplification
- Suicide PCR – Self-limiting amplification system48
1. Conventional PCR
- The Conventional PCR (also called end-point / traditional PCR) is the basic form of PCR in which amplification of target DNA is done through repeated cycles, and detection is performed only after all cycles have completed.
- A DNA template, primers (forward & reverse), dNTPs, buffer, Mg2+ and a thermostable DNA polymerase are required for Conventional PCR.
- Thermal cycling is carried out which consists of denaturation (high temperature), annealing (lower temperature), extension (intermediate temperature); these steps are repeated many times (commonly ~25-40 cycles).
- After amplification, the end-point analysis is performed by gel electrophoresis, whereby the amplified DNA product (amplicon) is separated on agarose gel and visualised (commonly via ethidium bromide) under UV light.
- Specificity is dependent on primer design, annealing temperature, reagent quality, and cycle parameters.
- Sensitivity is moderate; detection of small amounts of DNA is possible but quantification is inaccurate because measurement is done only at end, not during amplification.
- Simple equipment is used: conventional thermal cycler, electrophoresis apparatus.
- Limitations are present: non-specific amplification (primer dimers etc.), plateau effect (reaction efficiency drops after many cycles), risk of contamination during post-PCR handling.
- The Conventional PCR is used for applications like cloning, mutation detection, presence/absence assays, sequencing preparation, where quantitative real-time data is not essential.1234
2. High-fidelity PCR
- High-Fidelity PCR is defined as PCR in which a DNA polymerase with low error (high accuracy) is employed, so that misincorporation of wrong bases is minimized.
- A proofreading activity (3′→5′ exonuclease) is possessed by many high-fidelity polymerases, which permits removal of mispaired nucleotides that have been inserted incorrectly, before extension continues.
- The error rate is much lower than that of non-proofreading enzymes like Taq polymerase; for example fold improvements of 50- to >200-times have been reported.
- The error rate is influenced by reaction conditions (buffer composition, Mg2+ concentration, dNTP concentrations, annealing temperature), which must be optimized to retain fidelity in addition to yield.
- Template length / amplicon size tends to affect performance, longer targets are more error-prone, so enzyme selection and cycle number must be managed carefully.
- Amplifications required for downstream applications (cloning, sequencing, mutation detection) are made more reliable by High-Fidelity PCR, because the risk of having unwanted mutations (due to polymerase errors) is reduced.
- The fidelity is sometimes measured using assays like lacI phenotypic mutation assay, blue/white colony screening, next-generation sequencing (NGS) with molecular barcoding to separate background noise from true errors.
- Commercial High-Fidelity polymerases (e.g. “Q5”, “iProof”, Phusion etc.) are used. For instance iProof polymerase has an error rate ~4.4 × 10-7 in HF buffer, which is approx 50-fold lower than that of Thermus aquaticus (Taq) polymerase.
- Limitations are present: reactions are more expensive, sometimes slower (longer extension times), sensitivity to reaction components is higher, and it is possible that very low level errors still occur (especially for many cycles or when starting template is small) which must be considered.5678
3. Hot-Start PCR
- Hot-Start PCR is a variation of the PCR technique in which DNA polymerase activity is suppressed until a high temperature (usually the first denaturation step) is reached, so that nonspecific amplification is reduced.
- The DNA polymerase (or another essential component) is blocked or inhibited at room temperature, which prevents primer-dimers or mis-priming before cycling begins.
- Methods of inhibition include chemical modification of the polymerase, use of an antibody that binds to enzyme’s active site, aptamer-based blocking, or modified dNTPs or primers; some protocols delay addition of polymerase until after heating.
- The activation of polymerase is forced by heat, often during the first denaturation (≈ 95 °C), which causes the blocking agent (chemical / antibody / aptamer) to be removed / denatured, thereby the enzyme becomes active.
- Specificity is increased by Hot-Start PCR, because premature binding of primers (and thus non-specific amplification) is prevented during reaction set up, which improves yield of the correct amplicon.
- Sensitivity of detection is improved in many cases, because background noise (primer dimers, misprimed products) is lowered, so smaller quantities of target DNA can be more reliably amplified.
- Reaction set up at room temperature is made easier by Hot-Start PCR, because reagents are stable / inactive until heat activation, which reduces care required to avoid nonspecific binding during pipetting or mixing.
- Limitations are present: activation time is often required (first denaturation may need to be longer), sometimes cost is higher (antibodies / modified enzymes), and some residual low temperature activity (“leakiness”) may occur in some enzyme systems.9
4. Fast-cycling PCR
- Fast-Cycling PCR is a version of PCR in which the times for denaturation, annealing, extension (and sometimes initial / final steps) are reduced / shortened relative to conventional PCR which saves time.
- Specialized enzymes / polymerases with high processivity are required, which allow many nucleotides to be added quickly, so extension step durations can be shortened significantly without loss of performance.
- Thermal cyclers with fast ramp rates (rapid heating / cooling) are used, which reduce the time lost during transitions between temperature steps, so overall cycle times are decreased.
- Protocols are sometimes modified, which include combining annealing and extension steps into single step (two-step PCR), or reducing hold times at each step (denaturation, annealing, extension) to minimal effective durations.
- Amplicon (target DNA fragment) length influences how fast cycling can be done; shorter amplicons are more easily amplified with fast-cycling conditions, longer ones require more time or special optimization.
- Specificity and Sensitivity are maintained, when optimization is done properly (primer design, polymerase choice, buffer composition), so that fast-cycling does not lead to increase in nonspecific products or loss of yield.
- Time savings are substantial; full Fast-Cycling PCR runs may be completed in tens of minutes instead of 1-2 hours, depending on reagent / instrument capabilities.
- Limitations are present: not all enzymes tolerate shortened times / high ramp rates; robustness may be compromised if the template is complex (high GC content, secondary structures), or if primers are suboptimal; more optimization is required.1011
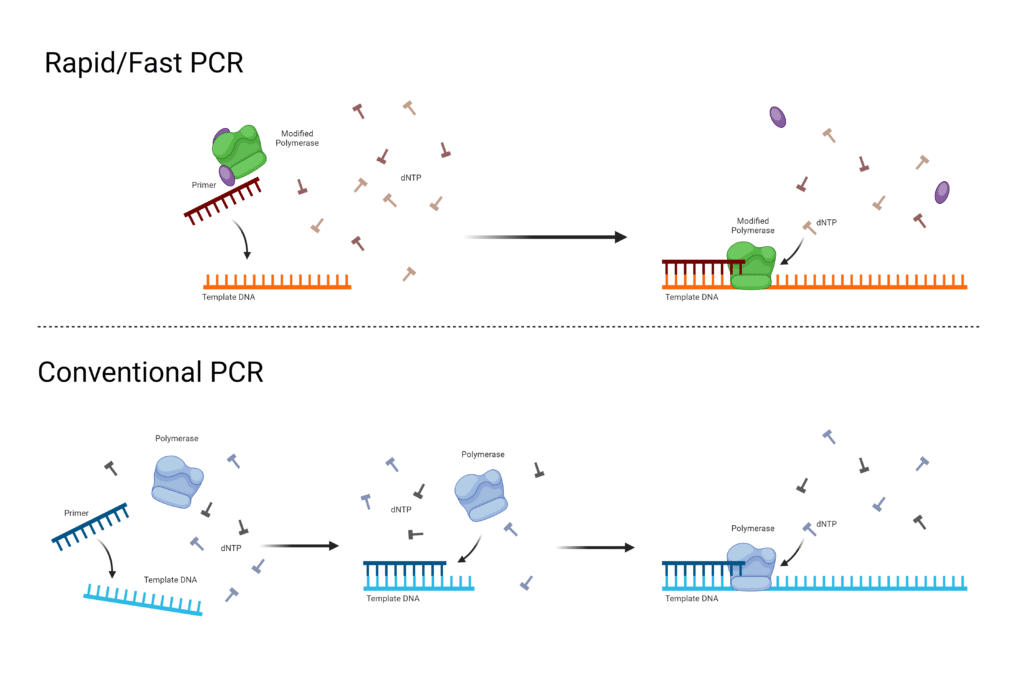
5. Touch down (TD) PCR
- Touchdown PCR (TD-PCR) is a modified PCR method in which annealing temperature is initially high, then gradually decreased over cycles, so that specificity is increased and non-specific binding is reduced.
- In early cycles the annealing temperature is set several degrees (often 5-10 °C) above the calculated melting temperature (Tₘ) of primers, which forces only high stringency primer-template pairing.
- In subsequent cycles the annealing temperature is reduced stepwise, by small decrements (e.g. 1-2 °C per cycle) until a lower, more permissive (but still specific) annealing temperature is reached.
- After the touchdown phase (high-to-lower temperature cycles), standard PCR cycles are often performed at the final annealing temperature, which yields further amplification of the correctly primed (desired) product.
- Specificity is improved because early amplification of correct template (with perfect primer match) is favored, and these early products compete out non-specific amplicons when lower annealing temperatures are later used.
- Sensitivity and yield are often enhanced by Touchdown PCR, because correct product accumulation is boosted, even if low amount of target template or borderline primer-template matching is present.
- TD-PCR is useful when problematic templates are used (for example with high GC content, secondary structure, or when primers are not perfectly optimized) or when non-specific amplification is seen with regular PCR.
- Limitations are present: longer protocol setup (because annealing temperature changes), possible requirement for more optimization, sometimes decreased yield in earliest cycles if annealing temperature is too high, which must be balanced.12
6. Real-Time PCR (qPCR)
- Real-Time PCR (also called qPCR or quantitative PCR) is a PCR variant in which amplification of DNA is monitored during the reaction, not only after its completion.
- A fluorescent reporter molecule is included in each reaction, which produces signal proportional to amount of amplified product, so that fluorescence is measured after each cycle to infer product accumulation.
- Specialized thermal cyclers equipped with fluorescence detection modules are required, which allow monitoring of amplification in closed-tube format so that post-PCR handling is minimized, and contamination risk is reduced.
- Quantification is enabled, so that starting quantity of target DNA (or after reverse transcription, RNA converted to cDNA) can be estimated with accuracy over a wide dynamic range.
- Two common detection chemistries are used: nonspecific DNA-binding dyes (e.g. SYBR Green) which bind to all double-stranded DNA; and sequence specific probes (e.g. TaqMan etc.), which bind only when matching their target sequence.
- The amplification plot is observed typically, with an exponential phase (where product roughly doubles each cycle) followed by non-exponential (plateau) phase when reagents become limiting.
- The quantification cycle (often called Cq, or Ct – threshold cycle) is defined as the PCR cycle number at which fluorescence exceeds a defined threshold above background.
- Relative quantification is performed by normalizing target gene signal to reference (housekeeping) gene signal, or absolute quantification is done by comparing to standard curves of known template amounts.
- Applications include gene expression analysis, detection & quantification of pathogens, measurement of genetic variants / copy number, biomarker discovery.
- Limitations are present: reagent cost is higher; primer/probe design must be more careful; amplification efficiency must be validated; inhibitory substances may affect fluorescence or polymerase activity; quantification may be inaccurate if reaction efficiency is not near ideal.13
7. Digital PCR (dPCR)
- Digital PCR (dPCR) is a PCR variation in which absolute quantification of nucleic acids is enabled by partitioning of the reaction mix into many small / micro-reactions, so that individual target molecules are amplified separately.
- In each partition (droplet, well, chamber etc.) either zero, one or more target molecules are present, and amplification is performed in each partition until endpoint, after which presence / absence of product is detected (binary read-out).
- The Poisson statistical model is used, which corrects for partitions that may contain more than one target molecule, so that absolute copy number (or concentration) is calculated.
- The sample is digitized by dividing into thousands to tens of thousands of partitions / droplets, which increases precision, because many small reactions reduce competition or bias between target and non-target sequences.
- Relative to qPCR, dependency on standard curves is eliminated, because quantification is derived from numbers of positive / negative partitions rather than cycle threshold comparisons.
- Sensitivity is greatly improved, which allows detection of rare mutations or low-abundance targets in presence of background non-mutant DNA.
- Tolerance to inhibitors is higher, because partitioning dilutes inhibitors in many partitions, reducing their overall effect on amplification in individual partitions.
- Amplification chemistries similar to those in qPCR (primers, probes, master mix) are used, only the format (partitioning + endpoint detection) is different.
- Limitations are present: cost is higher (due to consumables, instrumentation), throughput may be lower, and requirement for careful optimization is more.14
8. High-Resolution Melt (HRM) PCR
- High-Resolution Melt PCR (HRM) is a PCR-based technique in which the melting behavior of double-stranded DNA amplicons is monitored with high precision, so that small sequence variations are detected.
- The DNA region of interest is amplified by Real-Time PCR with inclusion of saturating DNA-binding fluorescent dye, which fluoresces when bound to double-stranded DNA but loses signal as melting proceeds.
- Precise heating (temperature ramp) is applied, with small increments (e.g. ~0.1-0.3 °C) during the melt phase, which allows high density data acquisition, so that melting curves are finely resolved.
- The melting curves are normalized and difference curves can be generated, which permit discrimination among homoduplex (identical DNA strands), heteroduplex (mismatched strands) or mutant/wild-type sequence variants.
- Sequence features such as GC content, length of amplicon, base composition / mismatches influence melting temperature (Tₘ) and curve shape, which are used in HRM to distinguish variants.
- Applications are mutation scanning, single-nucleotide polymorphism (SNP) genotyping, methylation analysis (e.g. methylation-sensitive HRM), zygosity testing, species or strain discrimination etc.
- The method is cost-effective compared to probe-based genotyping or sequencing when many samples require screening, because additional reagents / probes are minimized, and post-PCR handling is reduced.
- Limitations are present: quality of DNA template must be high, primers must be well designed, amplicon length must be small to moderate, instrument must support high thermal precision / stability and use of appropriate dyes; small sequence changes may still require confirmatory methods.1516
9. Reverse-Transcriptase PCR (RT-PCR)
- Reverse-Transcriptase PCR (RT-PCR) is a molecular biology technique in which RNA is first converted into complementary DNA (cDNA) by a reverse transcriptase enzyme, then the cDNA is amplified by PCR.
- The technique is used when RNA is the starting material, which allows detection / quantification of gene expression, viral RNA etc.
- Enzymes required include a reverse transcriptase (for RT step) and a DNA polymerase (for amplification), primers specific to the RNA target or general (e.g. oligo-dT, random primers) depending on application.
- One-step RT-PCR is performed, in which reverse transcription and PCR amplification are done in same tube/reaction mix.
- Two-step RT-PCR is used, in which the RT step is done first, cDNA is produced, then an aliquot of cDNA is used for PCR amplification.
- Amplification products may be detected by end-point analysis (e.g gel electrophoresis) or monitored in real time (if qPCR is combined) using fluorescent dyes / probes.
- Sensitivity is high, which allows detection of low-abundance RNA transcripts, small sample amounts.
- Specificity is dependent on primer design, enzyme fidelity, purity of RNA template, and conditions of RT step (temperature, inhibitors etc.).
- Limitations are present: RNA is labile (easily degraded), reverse transcription step may introduce variability, background signal / genomic DNA contamination may occur, quantification may be skewed if efficiencies are not ideal.17
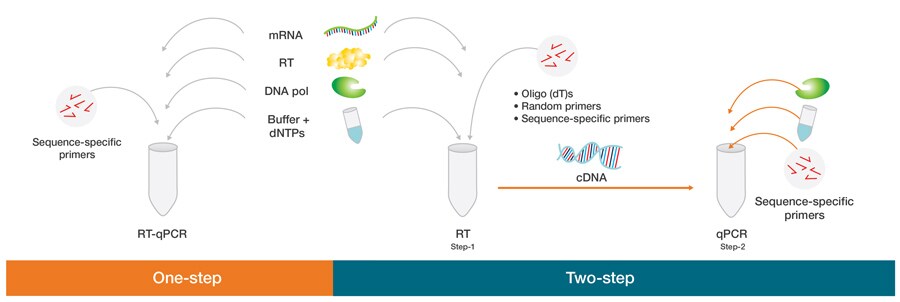
10. RT-qPCR – Real-time quantitative reverse transcription PCR
- RT-qPCR (Reverse Transcription Quantitative Real-Time PCR) is a combined molecular method in which RNA is converted into complementary DNA (cDNA) by reverse transcriptase, and thereafter amplification + quantification of that cDNA is done in “real time” by PCR.
- The starting material is RNA (total RNA or messenger RNA (mRNA)), which is reverse transcribed to cDNA, which serves as template for the PCR amplification.
- Fluorescent reporter molecules or probes are included, which allow detection of the amplified product after each cycle, so that quantification is done during the exponential phase, rather than at end-point.
- One-step RT-qPCR format is used when reverse transcription and quantitative PCR are combined in a single reaction (same tube / buffer), which reduces handling and contamination risk.
- Two-step RT-qPCR format is used when reverse transcription is done first separately, then an aliquot of cDNA is used for qPCR, which allows more flexibility (for example different primers, more optimization) and reuse of cDNA for multiple targets.
- Accuracy and sensitivity are high in RT-qPCR, which enables detection of low-abundance RNA transcripts, small changes in gene expression, or pathogen RNA detection.
- Wide dynamic range is provided, so that over several orders of magnitude of RNA concentration quantification is possible.
- Normalization is required, which usually involves internal reference genes (housekeeping genes) to control for variation in RNA amount, quality, or efficiency of reverse transcription.
- Limitations are present: enzyme efficiencies (both reverse transcriptase and DNA polymerase) must be validated, inhibitors or degraded RNA may affect result, and cost / reagent complexity is greater than simpler PCR.
- Applications are many: Gene expression profiling / quantification, pathogen detection (viral RNA quantification), validation of microarray results, disease biomarker studies, genetic testing etc.18
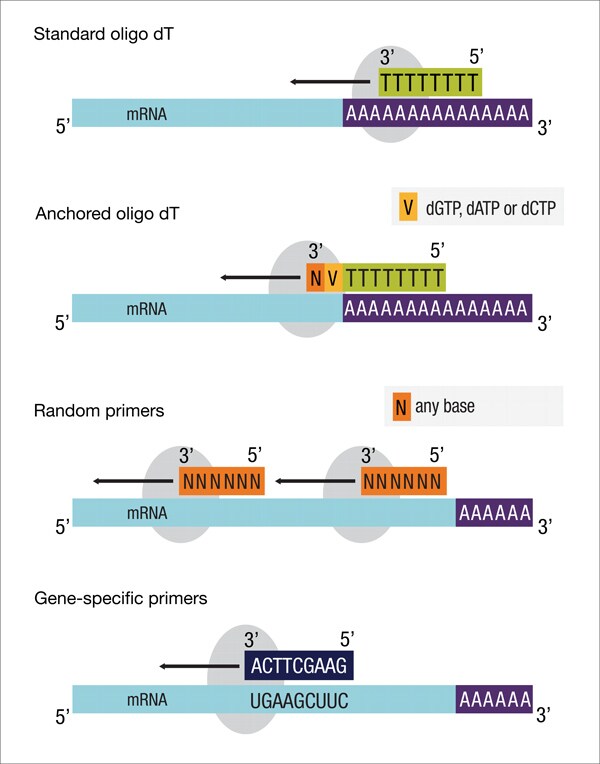
11. RNase H-dependent PCR (rhPCR) – RNA-specific amplification method
RNase H-dependent PCR (rhPCR) is a modification of standard PCR in which primers are blocked at 3′ end and activation is made dependent on RNase H2 cleavage, so that non-specific amplification is reduced.
The rhPrimers are designed to contain a single RNA base near the 3′ blocking group, which prevents extension by DNA polymerase until the block is removed.
A thermostable RNase H2 enzyme (often from Pyrococcus abyssi) is included, which recognizes the RNA:DNA heteroduplex after the primer hybridizes to the template, cleaves at 5′ side of the RNA base, removing the block, allowing DNA polymerase extension.
Since the blocking remains intact unless correct hybridization occurs, primer-dimer formation and mis-priming are strongly suppressed, which increases specificity of the PCR reaction.
In many protocols the rhPCR primers perform comparably to unmodified primers in amplification efficiency when RNase H2 is provided, but improved signal‐to‐noise (less background, fewer artifacts) is observed.
The enzyme’s thermal stability is important; RNase H2 must tolerate high temperatures used during PCR (e.g. denaturation steps), and its activity at lower temperature (room temp) must be minimal so that hot-start‐like behavior is conferred.
The effect of mismatches near the RNA residue is significant; mismatches reduce cleavage efficiency by RNase H2, which helps discrimination (single nucleotide polymorphisms, or variant sequences) and reduces off-target amplification.
Applications are many: SNP genotyping; detection of rare alleles; multiplex PCR where many primer pairs are used and unwanted primers interactions must be minimized; environmental DNA species discrimination; recovery of full-length variable regions (e.g immune receptor/TCR sequencing) etc.
Limitations / considerations are present: correct design of rhPrimers (blocker stability, position of RNA base) is required; enzyme concentration / buffer composition must be optimized; sometimes blocking group may be removed non-specifically by polymerases with 3′ exonuclease activity, or leaking may happen; costs are somewhat higher than simplest PCR.19
12. Multiplex PCR – Simultaneous amplification of multiple targets
Multiplex PCR is a PCR variant in which several different DNA target sequences are amplified simultaneously, by using multiple primer pairs in a single reaction tube.
With multiple primers present, primer design is critical, which requires optimization so that all primer pairs anneal effectively at similar annealing temperature, and that primer-dimer formation / cross-interaction is minimized.
The amplicon sizes (lengths in base-pairs) must be made different enough, or different probes / fluorescent labels must be used, so that distinct products can be differentiated (by gel electrophoresis or by fluorescence detection).
In many diagnostic / research applications, multiplex PCR is used for pathogen detection (several pathogens at once), SNP genotyping, mutation / polymorphism analysis, forensic profiling, GMO detection etc.
More efficient use of sample material is enabled, because limited input DNA/RNA can be tested for multiple targets in one reaction, reducing reagent consumption and time.
Throughput is increased by multiplex PCR, which makes processing many targets / many samples faster, so that more data can be obtained per run.
Specificity and sensitivity may be compromised if assay is not well optimized, because competition among primer pairs, unequal amplification efficiencies, or primer interactions reduce yield or produce non-specific bands.
Optimization is required strongly: primer concentration balancing, annealing temperature tuning, buffer / polymerase mixtures may be adjusted, and validation of each primer pair’s performance in multiplex context must be done.
Cost-benefit is present: although more optimization time & reagent testing is initially required, savings are made later in time / reagents / samples per test when many targets are routinely tested.20
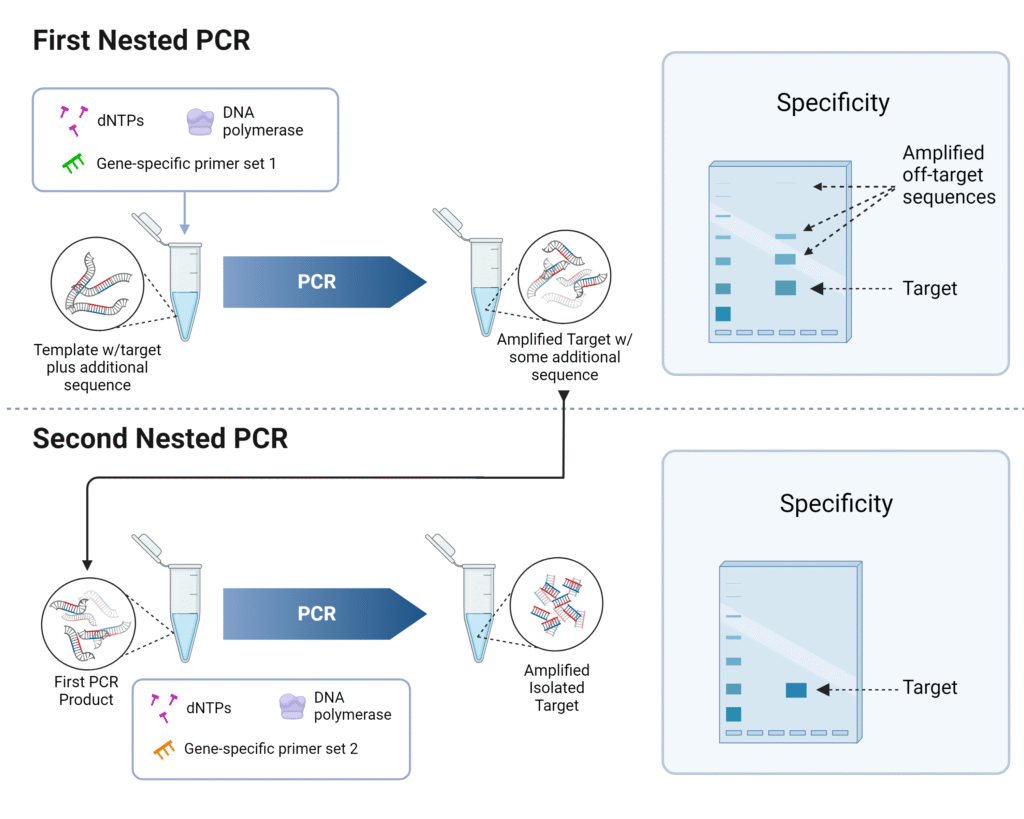
13. Nested PCR – Two-round amplification for enhanced specificity
Nested PCR is a PCR modification in which two successive rounds of amplification are performed, using two sets of primers, so that specificity and sensitivity are increased.
In first round, “outer” primers are used, which flank a broader region or include extra non-target flanking DNA.
In second round, “inner” or “nested” primers are used, which bind within the amplicon generated by the first PCR, so that only correctly amplified first-round product is efficiently further amplified.
With nested primers, non-specific products arising from mis-priming in first PCR are very unlikely to contain binding sites for both inner primers, so non-target amplification is reduced.
Sensitivity is enhanced by nested PCR, because low abundance target sequences may be amplified more reliably by the double amplification strategy.
More cycles may be run overall (first + second PCR), which allows small amounts of template to be detected, which however increases chance of contamination.
Primer design knowledge is required, because sequence information about the target and flanking regions must be known to design outer and inner primers properly.
Applications include detection of rare pathogens, low copy-number genes, degraded DNA (for example from formalin-fixed tissue), or when background (non-target DNA) is high.
Limitations are present: time is increased (two PCR rounds), cost is higher (extra primers, reagents, handling), contamination risk is elevated (because first round products are manipulated), and optimization of conditions is more demanding.21
14. Long-range PCR – Amplification of large DNA fragments (>5kb)
Long-Range PCR is a specialized PCR technique in which DNA fragments much longer than in standard PCR are amplified, which allows targeting of large genomic regions (e.g. >5 kilobases).
A special polymerase (or blends of polymerases) with high fidelity (proofreading capability) and high processivity is required, which helps to maintain accuracy over long extension lengths.
Amplification of large amplicons (for example 6.6 kb, 13 kb, 20 kb etc) is enabled when long-range PCR protocols are used, whereas routine PCR often fails for such lengths.
Buffer composition / reaction additives (enhancers) are employed, which reduce problems such as secondary structure, GC-rich regions, template degradation & depurination, which are more problematic when long templates are used.
Thermal cycling conditions are modified, which include longer extension times, sometimes lower extension temperature (e.g. 68 °C instead of 72 °C), shorter denaturation times, to reduce damage to the template and to keep fidelity high.
Template quality and integrity are critical, because fragmented or damaged DNA will impede amplification of long regions, resulting in drop-outs or truncated products.
Primer design is more demanding: primers must be specific, have melting temperatures (Tₘ) well-matched, minimal secondary structure, and the amplicon ends must be chosen to avoid problematic regions (high GC content, repeats etc.).
Application areas include sequencing of large genomic regions, mapping structural variation, cloning long genes (including introns), checking for large deletions or insertions, and generation of long probes or templates.
Limitations are present: yields may be lower, error rates might increase if fidelity is not very high, reaction failure risk is higher, optimization takes more time, and reagent/instrument costs may be greater.2223
15. Assembly PCR – Synthetic gene construction method
Assembly PCR is a method in which many short single-stranded oligonucleotides, having overlapping ends, are assembled into a longer DNA molecule by successive hybridization and extension, without needing a full template.
The oligonucleotides are designed so that adjacent ones overlap by a defined number of bases (for example ~20 bp overlaps), which ensures annealing / hybridization among them in correct order.
During early cycles, the overlapping oligos anneal to each other, and DNA polymerase extends them, so that partial fragments of different lengths are formed, gradually building up longer strands.
After assembly cycles, outer primers (which flank the entire desired full-length sequence) are added (or used) so that a conventional PCR amplification is performed, which selectively amplifies the full-length assembled product away from shorter incomplete fragments.
With Assembly PCR, synthetic genes (hundreds-to-thousands of base pairs) can be constructed de novo, even when no natural DNA template is available.
Sequence fidelity is dependent on oligo quality, polymerase fidelity, overlap design, and error correction steps, because synthesis and assembly errors (mismatches, deletions etc.) may accumulate.
The concentration of oligonucleotides, annealing temperature, cycle number during assembly must be optimized, because too low annealing temperature or too many oligos may cause mis-annealing or incomplete assembly.
Limitations are present: longer constructs are more difficult to assemble (due to competition, incomplete overlap, secondary structures), cost and error correction increase with size, and the process is more laborious than simple PCR of existing templates.24
16. Overlap extension PCR – Site-directed mutagenesis and gene fusion
Overlap Extension PCR (also called Splicing by Overlap Extension, SOE-PCR) is a PCR variant in which separate DNA fragments are fused together through overlapping ends, so that no restriction‐site is needed for joining.
In initial step, fragments are amplified separately, each having primers that introduce overhangs / complementary ends to adjacent fragments, which permit annealing between fragments in later stage.
After denaturation, overlapping regions of fragments are allowed to anneal, and DNA polymerase extends from the overlapping overhangs, thus creating fused hybrid fragments; outer primers are later used to amplify the full fused product.
The overlapping overhangs (usually at 5′ ends of primers) must be long enough (for example dozens of nucleotides) to ensure efficient and specific annealing / hybridization, which improves fusion efficiency.
A high-fidelity DNA polymerase is preferred, because fusion of fragments, especially large ones, requires accurate synthesis in overlap and extension regions so that unwanted mutations are minimized.
When multiple fragments are to be assembled, OE-PCR may be done in few steps: fragment amplification, overlap extension without outer primers, then final amplification with external primers.
Application is wide: chimeric gene construction, insertion of desired mutation or tag, promoter / terminator fusions, protein fusions, synthetic gene assembly.
Advantages are: no reliance on restriction enzyme sites (scarless fusions possible), flexibility in designing fused constructs, capacity for building large constructs (>20 kb) with proper polymerase and optimization.
Limitations / considerations are present: overhang design must be precise; concentration of primers/fragments must be balanced; background from incomplete fusion or mis-annealing may occur; reaction optimization (annealing temperatures, cycle numbers) is required.2526
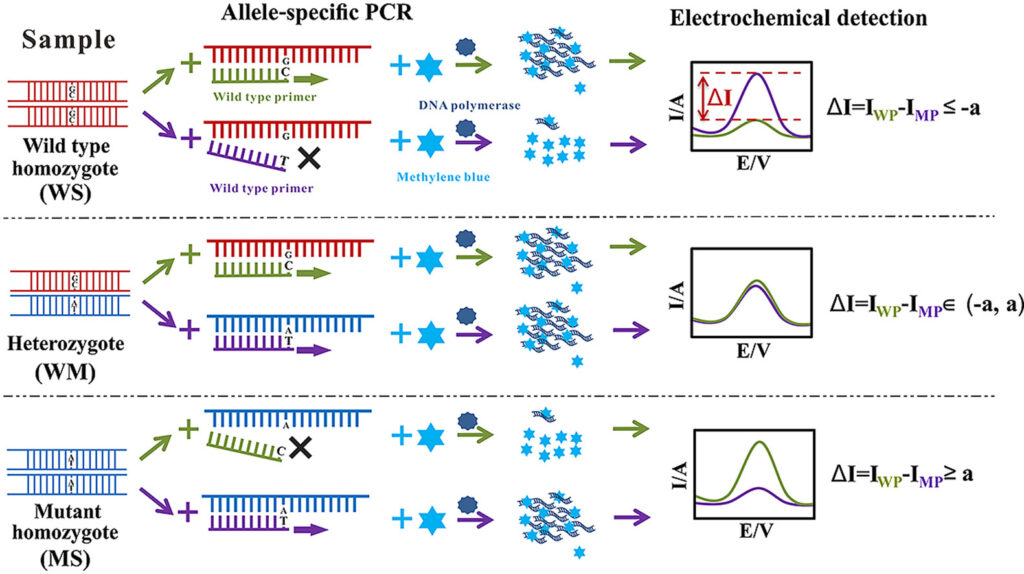
17. Allele-specific PCR
Allele-Specific PCR (AS-PCR) is a PCR method in which allele discrimination is achieved by using primers that allow amplification only when a specific allele (variant) is present.
In AS-PCR, primers are designed so that their 3′-end base (or near it) matches exactly the nucleotide variant (SNP or mutation) of interest, which causes extension only when perfect match is present.
With two allele-specific primers (one for wild-type allele, one for mutant allele) and a common (or reverse) primer, separate reactions (or multiplexed) are performed to test which allele is present.
Specificity is enhanced by introducing mismatches near 3′ end (besides the SNP base) to destabilize primer binding when allele is non-matching, which reduces non-specific amplification.
Detection of the allele-specific products is done by typical PCR readouts: gel electrophoresis of amplicon sizes, or by use of fluorescence/probe or melting curve analysis if real-time or labelled primers are used.
Sensitivity is moderate to high, which allows detection even when variant allele is present at low proportion, provided that reaction conditions are highly optimized.
Limitations are present: primer design must be precise, non-specific amplification or false positives/negatives may occur if mismatch discrimination is weak, and reaction optimization (temperature, primer concentration, buffer etc.) is required.
Applications include genotyping of single nucleotide polymorphisms (SNPs), detection of point mutations, pharmacogenetics, diagnosis of genetic diseases, and research in population genetics etc.27
18. AFLP PCR
AFLP PCR (Amplified Fragment Length Polymorphism PCR) is a molecular/genetic fingerprinting method in which genomic DNA is digested with restriction enzyme(s), adapters are ligated, then a subset of fragments is selectively amplified by PCR, so that polymorphisms are detected. Wikipedia+2NCBI+2
With two different restriction enzymes (one “rare-cutter”, one “frequent-cutter”) DNA is cut into fragments, which generates many DNA pieces of varying lengths and ends.
Adaptors (double-stranded oligonucleotides of known sequence) are ligated to ends of restriction fragments, which provide primer binding sites for subsequent amplification.
Selective PCR amplification is performed with primers that match the adaptor plus restriction site plus additional selective nucleotide(s) (often 1-3 extra bases) at the 3′ end, which reduces the complexity by amplifying only a subset of fragments.
The amplified fragment set is separated (by gel electrophoresis or capillary electrophoresis) and visualized, which results in a band / peak pattern (“fingerprint”) that reflects presence / absence of many polymorphic fragments.
Genetic variation is inferred by scoring of bands (or peaks) as present (1) or absent (0), which allows comparison among individuals, strains, populations, etc.
High sensitivity is achieved by AFLP, which enables detection of many polymorphisms even when little prior sequence information is known about the organism.
Robustness and reproducibility are usually good, when DNA quality is high, enzymes work well, and selective primers are carefully designed and optimized.
Limitations are present: scoring of bands may be ambiguous; dominance (presence/absence) markers do not always distinguish heterozygotes; reproducibility across labs / gels / instruments may vary; high-throughput instrumentation may be required for large fragment sets.
Applications include population genetics; strain / variety fingerprinting; phylogenetic studies; mapping; detection of genetic variation among individuals when full genome sequence is not available.28
19. VNTR PCR
VNTR PCR (Variable Number Tandem Repeat PCR) is a PCR-based technique in which a region of DNA that contains tandemly repeated sequences (VNTRs) is amplified, so that allelic variation in repeat number can be detected.
The repeat unit in a VNTR is generally longer (often ~10-100 base pairs) than in microsatellites, which makes the amplicon size variable between individuals depending on how many repeats are present.
With primers designed to flank the VNTR region, amplification is performed by PCR, so that the fragment size (repeat + flanking regions) can be measured (by gel electrophoresis or capillary electrophoresis) to infer number of repeats.
Genetic variation is revealed because different individuals/strains carry alleles with different number of tandem repeats; those length polymorphisms act as markers.
In forensic / population genetic applications, VNTR PCR is used, because its discrimination power is high when multiple VNTR loci are used; matching of VNTR patterns among loci yields individual / strain-specific profiles.
Sensitivity and specificity depend on primer design (flanking regions must be conserved), DNA quality, PCR conditions; alleles with very large repeat number can produce larger amplicons which may be more difficult to amplify or resolve.
Limitations are present: large VNTRs yield large PCR products which may amplify poorly; stutter bands or slippage by polymerase can cause artefacts; accurate sizing requires good resolution (high quality gels or capillary systems).
The method is widely used in molecular typing of microorganisms, genetic diversity studies, parentage / pedigree analysis, human identification (DNA fingerprinting) and epidemiological tracing.29
20. ISSR PCR
Inter-Simple Sequence Repeat PCR (ISSR-PCR) is a molecular marker technique in which primers based on simple sequence repeats (microsatellites / SSRs) are used in PCR to amplify the DNA regions between adjacent repeat loci, so that multilocus patterns / fingerprints are produced.
Primers of length ~16-25 base pairs which include microsatellite motifs (di-, tri-, tetra-nucleotide repeats) are used, often anchored at 3′ or 5′ ends with one to few non-repeat bases, so that binding specificity is improved.
Amplification of multiple loci is done in single reaction (single primer / pair) so that many inter-SSR regions are amplified, producing bands of various sizes when resolved by gel electrophoresis or capillary systems.
High polymorphism is observed among the ISSR markers, because inter-SSR regions vary in length / sequence among individuals or strains, which yields detectable differences in banding patterns.
Reproducibility is relatively good when DNA quality is high, primers are well designed, annealing temperatures are optimized, which makes ISSR useful where SSR or AFLP or RAPD might have limitations.
The technique is cost-effective, because no prior genome sequence is strictly necessary to design primers, and the same primers may work across related species; and labor / reagent costs are moderate.
Applications are genetic diversity studies, population structure analysis, phylogenetic relationships, cultivar / germplasm characterization, taxonomy, conservation genetics.
Limitations are present: dominant markers result (presence/absence) so heterozygosity may not be distinguished; large bands may amplify poorly; smeared / faint bands may reduce clarity; optimization of PCR conditions (annealing temp, primer concentration, cycle number) is needed.30
21. Methylation-specific PCR (MSP)
Methylation-specific PCR (MSP) is a technique in which DNA methylation status (methylated vs unmethylated) is assayed by PCR, after bisulfite conversion of DNA.
With bisulfite treatment, unmethylated cytosines are converted into uracil (ultimately read as thymine in PCR), whereas methylated cytosines (5-methylcytosine) remain unchanged.
Two sets of primers are designed: one set specific for the methylated version of the target after bisulfite conversion, the other specific for the unmethylated version.
Amplification is performed separately with each primer set, under conditions that discriminate well between perfectly matched vs mismatched DNA-primer interactions, so that only the correctly matching template is amplified.
The presence or absence of PCR product (from methylated or unmethylated primers) is detected (commonly by gel electrophoresis) to determine methylation status at specific CpG sites / CpG islands.
Sensitivity is high; detection of small amounts of methylated DNA among a background of unmethylated DNA is possible.
Primer design is critical which involves inclusion of several CpG sites in the primer binding region (especially near 3′ end), so that discrimination between methylated vs unmethylated is strong.
Quality of bisulfite conversion is essential; incomplete conversion leads to false positives (unconverted unmethylated cytosines being interpreted as methylated).
Limitations are present: qualitative (presence/absence) rather than fully quantitative in many implementations; ambiguity may arise if mixed methylation patterns; DNA degradation during bisulfite treatment may reduce yield; design of primers for dense CpG regions may be challenging.31
22. SSP-PCR
SSP-PCR (Sequence Specific Primer PCR or Single Specific Primer-PCR depending on context) is a PCR method in which primers that are specific for one allele / sequence variant are used, so that only exact matching sequences are amplified.
Primers must be designed so that mismatches (especially at 3′ end) prevent amplification when non-target allele is present, which ensures high specificity.
In HLA typing, SSP-PCR is commonly used, multiple primer pairs (each allele-specific) are used, so that presence / absence of bands indicates which allele(s) are present in sample.
Single Specific Primer-PCR (a variant) is performed when only one end of sequence is known; unidirectional “genome walking” into unknown region is made possible by SSP-PCR, which allows adjacent unknown DNA to be amplified from known region.
Amplification is carried out under stringent PCR conditions, which reduce non-specific priming, because only perfectly matching primer / template combinations allow extension.
Detection is generally by gel electrophoresis (presence / absence of band) or by visualization of each allele-specific reaction, which yields yes/no signal per allele.
Sensitivity and specificity are high, when primers are well designed and reaction conditions are optimized, which is why SSP-PCR is widely used in genetic typing, HLA typing, allele discrimination etc.
Limitations are present: many separate reactions (or many primer pairs) may be required to test multiple alleles; false positives / negatives may occur if primer binding is imperfect; cost / labor of primer design / validation is higher.32
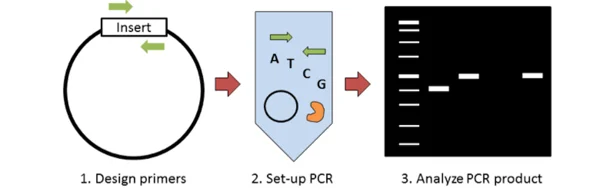
23. Colony PCR
Colony PCR is a screening PCR method in which bacterial (or yeast) colonies are used directly as source of template without needing plasmid purification, so that clone verification is sped up.
After transformation and plating, a single colony is picked (by toothpick, pipette tip etc.), which is transferred into PCR master-mix to supply DNA template.
Lysis of cells is performed during initial heating / denaturation in PCR (or by simple boil / other crude lysis), which allows plasmid / genomic DNA to become available for amplification.
Primers are designed in various ways: insert-specific primers, backbone or vector flanking primers, or orientation-specific primers (one in insert, one in backbone), which give information about presence, size, or orientation of the insert.
Amplification is carried out by standard PCR cycles, though sometimes modifications (longer lysis step, adjusted annealing temperature) are used to accommodate cellular debris / impurities.
After PCR, products are analysed (commonly by agarose gel electrophoresis), which permits identification of colonies carrying the desired insert by correct band size(s).
Time and cost are saved, because plasmid minipreps and restriction digests are avoided for many colonies, which reduces labour and reagent usage.
Specificity and sensitivity are moderate to high, when colony PCR is well optimised, but false positives or negatives may occur if too many cells are picked, debris inhibits PCR, or primers are not well designed.33
24. Single cell PCR
Single-cell PCR is a technique in which the genetic material (DNA or RNA) from a single cell is amplified by PCR, so that analysis of that one cell can be done.
With single cell PCR, the amount of starting template is extremely small (often picogram levels), which necessitates very sensitive reagents and careful handling to avoid loss or contamination.
Cell isolation is performed first, by micro-dissection, micromanipulation, fluorescence-activated cell sorting (FACS), or other single cell capture techniques, which ensure that only one cell is used per reaction.
Lysis of the single cell is done so that its DNA or RNA is released; for RNA, reverse transcription is done to convert RNA into cDNA before PCR amplification.
Whole genome amplification (WGA) or whole transcriptome amplification (WTA) may be included, when multiple loci or many genes are to be assayed from that one cell, so that enough template is generated for downstream PCR(s).
Amplification is carried out by PCR, often with modification / optimization of cycle numbers, primer concentrations, polymerase choice (high fidelity) and reaction volumes, so that drop out (loss of some alleles) or bias (unequal amplification) is minimized.
Quantitative PCR (qPCR) is sometimes used (Single-cell RT-qPCR) when measurement of gene expression (RNA levels) is required, which gives dynamic range and quantification rather than just presence/absence.
Sensitivity is very high, which enables detection of rare transcripts, low-copy genes, or mutations in a single cell, which bulk-cell PCR would mask.
Limitations are present: amplification bias may occur (some alleles amplified more than others), allelic dropout may happen (one allele fails to amplify), contamination risk is high because of extremely low input, and reproducibility / accuracy depend on extreme optimization.34
25. In situ PCR
In situ PCR is a molecular method in which amplification of specific DNA or RNA sequences is done within intact cells or tissue sections, so that spatial / anatomical localization of target nucleic acid is preserved.
With tissue fixation and permeabilization steps, the nucleic acids are made accessible (by proteolytic digestion etc.), which allows primers and polymerase to enter the cells / slide and amplify target sequences in situ.
The amplification product is detected either by incorporation of labelled nucleotides during PCR (direct in situ PCR), or by subsequent hybridization of probes (indirect in situ PCR) or via histochemical / immunohistochemical methods.
Specificity / sensitivity are high, especially for low-copy genes, when all preparatory steps (fixation, permeabilization, amplification) are well controlled.
With formalin-fixed, paraffin-embedded tissue or other archival samples, nucleic acid quality / preservation may be poor, which reduces efficiency of amplification and increases background or false positives.
Artefacts may be introduced by diffusion of PCR products out of cells / tissue, or by non-specific incorporation of labelled nucleotides into fragmented DNA undergoing repair; appropriate negative controls are required.
In situ RT-PCR (reverse transcription PCR) is used when RNA targets (mRNA etc.) are to be detected in tissue sections, so that gene expression (at cellular level) is mapped.
The technique is useful for diagnostic pathology, detection of viral infections in tissue, mapping gene expression in developmental biology, or detecting gene rearrangements / translocations with preserved morphology.
Limitations are present: quantification is difficult; tissue morphology / fixation may hinder reagent access; risk of background signal is higher; handling is more tedious than for ordinary PCR; optimisation is laborious.35
26. Solid phase PCR
Solid-Phase PCR (SP-PCR) is a method in which one or both primers are immobilized (attached) onto a solid support (surface), so that DNA amplification occurs with at least one primer bound to that surface rather than both in solution.
With the primer immobilized (often via its 5′ end) and the 3′ end left free, extension by DNA polymerase is permitted when template / free solution-phase primer anneal occurs.
On solid surfaces such as glass, beads, microarrays or chip surfaces, the bound primer must be stably attached, which requires covalent linkage or other strong binding chemistry that survives repeated heating / cooling cycles.
Amplification efficiency is usually lower compared to conventional (solution-phase) PCR, because mass transport, steric hindrance, reduced primer mobility / availability of template to immobilized primer are limiting factors.
Specificity is benefitted by immobilization, because unintended interactions (primer dimers etc.) are reduced when one primer is anchored; signal-to-noise may be improved.
Denaturation, annealing, and extension steps are adapted so that the immobilized primer retains activity, and that the solid support does not degrade or lose bound oligos; thermal stability of attachment must be ensured.
Applications are many: microarray-based target detection; generation of immobilized amplicons for sequencing or SNP detection; diagnostic platforms where detection on surface is needed; potentially point-of-care where washing / signal capture is facilitated.
Limitations are present: lower yield; slower amplification; possibly higher cycle numbers needed; accessibility of template to the surface-bound primer can be reduced; design & optimization of surface chemistry, primer density, orientation, temperature cycling are more demanding.36
27. COLD PCR
COLD-PCR (Co-amplification at Lower Denaturation Temperature PCR) is a modified PCR protocol in which minority/variant alleles are preferentially enriched over wild-type alleles, so that low-level mutations become more detectable.
The principle is based on the existence of a critical denaturation temperature (Tc), which is lower than the melting temperature (Tm) of the target DNA / template; at Tc the heteroduplex DNA (variant + wildtype mismatched duplex) is more easily denatured, whereas homoduplex wildtype DNA remains double-stranded.
With setting of denaturation step at Tc rather than full denaturation, selective denaturation of mutant (or mismatched) DNA is done, which leads to their preferential amplification in subsequent cycles.
Fast COLD-PCR and Full COLD-PCR are two formats; in Full format intermediate annealing + hybridization (to allow heteroduplex formation) are included, whereas in Fast format some steps are skipped to speed up, although sensitivity / universality of mutation enrichment may be reduced.
Mutations at any position (not just specific known ones) may be enriched, irrespective of mutation type (substitutions, small insertions/deletions), when the method is optimized appropriately.
Amplification products after COLD-PCR are often analysed by downstream methods: Sanger sequencing, pyrosequencing, high-resolution melting, or other mutation detection assays, so that enriched variant alleles are more clearly visible above background.
Sensitivity is greatly increased; fold-enrichment of 10- to 100-fold has been reported for low-abundance mutations in mixtures of wildtype/variant DNA.
Precision of Tc (denaturation temperature) control is crucial, because small deviations (± ~0.3 °C or less) may reduce enrichment, or allow wildtype homoduplexes to denature and reduce specificity.
Limitations are present: sequence length of amplicon often must be kept relatively short (e.g. < ~200 bp) for good discrimination; some mutation types may not shift Tm noticeably; polymerase errors or inefficiencies may interfere; optimization required for each target.37
28. Asymmetric PCR
Asymmetric PCR is a PCR variant in which one DNA strand (single-strand) is preferentially amplified over its complementary strand, so that ssDNA (single stranded DNA) is produced.
A primer pair is used, but one primer (the excess primer) is provided in much higher concentration than the other (limiting primer), which causes early exponential amplification of both strands, then when the limiting primer is exhausted, linear amplification of just the strand primed by the excess primer occurs.
After the limiting primer is used up, the reaction proceeds slowly (linearly) rather than exponentially, which requires additional PCR cycles to accumulate sufficient single-strand product.
Primer design is important: melting temperature (Tₘ) of primers must be considered, especially for the limiting primer, to ensure annealing is still efficient even when its concentration falls; mismatch or poor annealing reduces yield.
Optimization of reaction conditions (primer ratio, cycle number, annealing temperature, template concentration, polymerase choice) is required, because yield of ssDNA is lower and artifacts / nonspecific amplification may increase.
LATE-PCR (Linear-After-The-Exponential PCR) is an improved form of asymmetric PCR, in which the limiting primer is chosen to have a higher Tₘ than the excess primer, so that when its concentration drops the reaction still works efficiently.
Applications include generation of single-strand DNA for hybridization probes, DNA sequencing, aptamer library preparation (SELEX), allele discrimination, microarray hybridization etc.
Limitations are present: yield is generally lower than symmetric (regular) PCR; efficiency and reproducibility are sensitive to exact primer ratios and reaction parameters; more PCR cycles may lead to more nonspecific products; single strand product may require purification.38
29. LATE PCR
LATE-PCR is a modified version of asymmetric PCR in which primers are used at unequal concentrations, but with novel design of melting temperatures so that high efficiency & specificity are maintained.
The limiting primer (in low concentration) is designed to have a concentration-adjusted melting temperature (Tₘ^L) at least equal to or greater than that of the excess primer (Tₘ^X), which reduces loss of amplification efficiency in early cycles.
In early cycles both primers are present, so exponential amplification of both strands is performed, similarly to symmetric PCR, which allows good initial amplification of double-stranded product.
Once the limiting primer is depleted, the reaction is switched to linear amplification, during which mostly the strand primed by the excess primer is produced, so single-strand DNA accumulates.
Primers must be designed carefully, which includes ensuring that Tₘ values are adjusted to account for difference in concentrations, and that primer binding sites avoid regions that may misprime or form secondary structure.
With LATE-PCR, more predictable kinetics are achieved, because the exponential phase is preserved and the abrupt switch to linear phase reduces plateau variability that is seen in traditional asymmetric PCR.
Quantitative real-time detection is facilitated, since single-strand product produced in linear phase can be probed (with probes or molecular beacons) with low background and with improved signal stability among replicate samples.
Applications include detection & quantification of low abundance DNA / RNA targets, single-cell genetic diagnostics, multiplex assays, viral detection, where single-strand product or strong signal with minimal noise is useful.
Limitations / considerations are present: more optimization is required (especially primer concentrations, Tₘ adjustment), excess cycles may be required to accumulate detectable single-strand product; probe design must accommodate the switching; sample quality, template integrity etc. exert strong effect.39
30. Inverse PCR
Inverse PCR (iPCR) is a PCR variant in which DNA flanking regions of a known internal sequence are amplified, although primers for those flanking regions are unknown.
A DNA sample is first digested with a restriction enzyme (often a 6- to 8-base cutter) which cuts outside / around the known region so that fragments containing the known + unknown flanking DNA are obtained.
With ligation at low DNA concentration or via specific reaction conditions, the restriction fragments are circularized (self‐ligated), which yields circular DNA molecules (loops) that bring together flanking unknown ends adjacent.
Primers are designed to match the known internal sequence, and are oriented such that extension proceeds outward (away from each other) across the ligated junction, into the unknown flanking regions.
Amplification is performed using the circularized DNA as template, which allows unknown outside sequences to be copied because the outward primers now have continuous template across the ligation junction.
The resulting PCR product contains the known “core” region plus adjoining flanking sequences, which can be sequenced and compared to database to identify insertion sites, integration sites, or genome walking.
Applications include identification of retroviral / transposon integration sites, mapping of unknown chromosomal DNA adjacent to known sequence, cloning of regulatory / promoter regions upstream of genes, and site‐directed mutagenesis on circular DNA (e.g plasmid mutagenesis).
Limitations are present: reasonable size of restriction fragment is needed (too large fragments may circularize inefficiently or amplify poorly), choice of restriction enzyme is important (must not cut within known core region ideally), and careful ligation / template preparation required.40
31. TAIL-PCR
TAIL-PCR is a PCR method in which unknown DNA sequences flanking a region of known sequence are amplified, so that adjacent unknown genomic DNA is recovered.
In this method nested / insertion-specific primers are used together with “arbitrary degenerate” (AD) primers, which are less specific and bind at multiple sites, so that one primer of a pair targets the known region, and the other (AD) binds somewhere in unknown flanking DNA.
Thermal cycling is arranged in several phases (primary, secondary, tertiary), which include alternating high stringency annealing cycles (for specific primer binding) and low/reduced stringency cycles (for AD primer binding) to permit flanking region priming, which yields specific-plus-non-specific products in early cycles, but specificity increases in nested rounds.
AD primers are designed with lower melting temperature (Tₘ) than the specific primers, which ensures that during low stringency cycles the AD primers can bind to many possible sites, but in high stringency cycles only specific primers dominate.
In secondary and tertiary PCR rounds nested specific primers (inner ones, closer to the known region) are used, which improve specificity by preferentially amplifying products that include the known target region plus flanking DNA, and by suppressing non-specific amplicons.
With TAIL-PCR, flanking regions adjacent to insertion sites (e.g. of T-DNA, transposons) are isolated; promoter / regulatory regions upstream of genes are recovered; and genome‐walking is facilitated.
High-efficiency versions (hiTAIL-PCR) have been developed, which optimize primer design and thermal cycling, to yield larger flanking amplicons and higher success rate (>90% in some plant lines) for unknown flanking sequence recovery.
Limitations are present: non-specific amplification may occur (especially by AD primers binding at many genomic sites), multiple rounds required which increase risk of contamination, unknown region size may limit amplification length, nested primer design is required and prior known sequence must be available.41
32. Ligation-mediated PCR
Ligation-mediated PCR (LM-PCR) is a technique in which a DNA fragment of interest having one known end is amplified by ligating an adapter (linker) to the unknown end, and then using primers against the known region and the adapter.
In this method, DNA is first digested (or sheared) to generate fragments, which after ends-preparation (blunt or sticky) are ligated with a known sequence adapter, which serves as binding site for the adapter-specific primer.
With a primer specific to the known DNA region (internal primer) and another primer specific to the adapter, PCR amplification is performed, which allows recovery of sequence adjacent to the known region.
Quantitative in vivo footprinting is a major application, which uses LM-PCR to detect sites of protein-DNA interaction (protected from cleavage) or methylation status, or DNA damage / lesion mapping.
Sensitivity is high, which permits detection of low abundance DNA fragments (adjacent to known region) even when only one end is known.
Specificity is enhanced by using nested primers (inner primers in subsequent rounds) and by controlling ligation and adapter design to avoid background amplification.
Linkers / adapters must be designed so that they ligate efficiently and do not self-ligate excessively; and PCR primers must be matched to the adapter plus known sequence, with considerations of melting temperature, GC content etc.
Limitations are present: multiple steps (digestion, ligation, purification) increase risk of sample loss or contamination; some fragments may ligate poorly or be too long for efficient amplification; size of unknown flanking region that can be recovered is often limited (commonly < ~200-350 bp though improvements exist).
Applications are genome walking, mapping of integration sites (e.g., transposons, viruses), identification of regulatory regions / promoters upstream of known sequence, DNA methylation or footprinting studies.42
33. Alu PCR
Alu PCR is a DNA fingerprinting method in which many genomic loci flanked by Alu repetitive elements are amplified simultaneously, so that polymorphisms / insertions / deletions or length variation between Alus are detected.
With primers complementary to Alu sequences (often two “Alu-specific” primers), inter-Alu regions (between two Alu repeats) are amplified, which yields a complex pattern of fragments of differing sizes.
Amplification products are analysed by capillary electrophoresis or gel electrophoresis, which separates the fragments by size, so that sample-to-sample variability appears as different band / peak patterns.
Genetic polymorphism is revealed, because Alu insertions (presence / absence), deletions, or mutations in or near Alu elements, or length differences in DNA between Alus cause variation in PCR fragment sizes among individuals.
Sensitivity for human DNA detection is high, because Alu elements are very abundant in the human genome (over 1 million copies), which gives many potential primer binding sites / many inter-Alu fragment templates.
Quantitative or semi-quantitative usage is possible, for example to detect small amounts of human DNA in mixed samples (e.g. among rodent DNA), by using Alu-based qPCR assays which are designed to avoid cross-amplification.
Limitations are present: complexity of fragment pattern may hinder interpretation; non-specific amplification / background may occur; degraded DNA may reduce yield, especially for large inter-Alu fragments; primer design must be optimized to avoid bias or artefacts.43
34. Repetitive sequence-based PCR
Repetitive Sequence-Based PCR (rep-PCR) is a molecular typing/fingerprinting method in which primers complementary to repetitive DNA elements dispersed in a genome are used, so that strain or species specific fingerprint patterns are produced.
In rep-PCR, repetitive elements such as REP (Repetitive Extragenic Palindromic), ERIC (Enterobacterial Repetitive Intergenic Consensus), BOX sequences etc are targeted, which are interspersed in bacterial genomes and provide many binding sites for primers.
Throughout the rep-PCR reaction, multiple PCR products of varying lengths are amplified, depending on the distances between repetitive elements (primer binding sites), so that a band / peak pattern (fingerprint) is generated.
With genomic DNA from different strains, the banding patterns are compared (by gel electrophoresis or capillary electrophoresis) which allows discrimination of strains / isolates based on similarity / differences in their repetitive-element distributions.
High throughput is enabled, because only one PCR per sample (or a small number with different primer sets) is needed, which speeds up screening of many isolates.
Specificity and reproducibility depend on primer selection, PCR conditions (annealing temperature, extension time), DNA quality and stability of repetitive sequences in the genome, which must be controlled to get reliable fingerprints.
Sensitivity is moderate; rep-PCR can detect differences among closely related strains, but small mutations (SNPs) not affecting repetitive element locations may not be resolved.
Lower cost is achieved compared to methods like full genome sequencing or PFGE (pulsed-field gel electrophoresis), because fewer reagents, less labour, simpler equipment (regular PCR + gel electrophoresis) are needed.
Limitations are present: fingerprint patterns can be complex to interpret; inter-laboratory reproducibility may vary; band scoring and gel resolution affect discrimination; repetitive elements may be absent or limited in some species, reducing utility.44
35. Nanoparticle-Assisted PCR (nanoPCR)
Nanoparticle-Assisted PCR (nanoPCR) is a PCR variant in which nanoparticles (NPs) are added into the PCR reaction, so that amplification sensitivity / specificity / speed are enhanced.
With metal nanoparticles (e.g. gold nanoparticles, AuNPs) often used, which provide improved thermal conductivity, so that heating/cooling transitions are more efficient, reaction kinetics are improved.
Interaction between NPs and PCR components is involved; for example adsorption of polymerase, primers or single-stranded DNA is mediated by NP surfaces, which modulates the reaction in favor of specific amplification and suppresses non-specific / mismatched primer binding.
In standard nanoPCR protocols optimal concentration, size, and type (metal, oxide, carbon-based etc.) of nanoparticle are determined, which ensures that enhancement (sensitivity / detection limit) is maximized without inhibition of PCR by too much NP.
Sensitivity is increased: in studies (e.g. detection of viruses like pseudorabies, bovine respiratory syncytial virus) nanoPCR has been shown to detect ~10- to ~1000-fold lower copy numbers compared to conventional PCR.
Specificity was benefitted, because nonspecific amplification / background bands were reduced, when nanoPCR was optimized, which helps distinguish target vs non-target very well.
Time saving has been reported; annealing / extension times have been reduced, and reaction efficiency improved, when NP-assisted systems are used under optimized thermal cycling and NP concentration.
With challenging samples (low template amount, degraded DNA or presence of inhibitors), nanoPCR has been applied to improve detection (i.e. overcoming inhibition) in clinical / field / veterinary diagnostics.
Limitations / considerations are present: nanoparticle concentration must be optimized carefully; NP size / type may affect results (some NPs may inhibit PCR if too large or at high conc.); potential effects on enzyme stability / fidelity must be checked; reproducibility between labs may vary.45
36. Miniprimer PCR
Miniprimer PCR is a variant of PCR in which very short primers (≈ 9-10 nucleotides, “miniprimers”) are used, which allows detection of more divergent sequences than with standard longer primers.
An engineered polymerase (e.g. S-Tbr, a thermostable enzyme modified to work with short primers) is employed, which permits extension from miniprimers with acceptable specificity and yield.
With primers shortened gradually from typical ~20-nt length to ~10-nt, annealing temperatures are optimized (lowered) to permit binding, which ensures that miniprimers can hybridize despite reduced complementarity.
The method has been applied to environmental samples (soil, microbial mats) for 16S rRNA gene amplification, which provided more novel / deeply divergent sequences than standard PCR primers, suggesting that many taxa may be missed by conventional methods.
Specificity / reproducibility are preserved when miniprimer PCR is well-designed, although background amplification / non-target binding is a risk because short primers bind more broadly; optimization of primer design, annealing temperature, enzyme choice etc. is needed.
Sensitivity is moderate to high, in that even low abundance or rare divergent 16S sequences were detected; yield is somewhat lower than standard PCR in some cases.
Limitations are present: primer binding promiscuity (non-specific annealing) may increase; ability to discriminate among very similar sequences may be reduced; amplification bias may occur; short primers may not be usable for some target regions with secondary structure or low complexity.
Applications include microbial diversity studies, environmental DNA surveys (to reveal novel lineages), genotyping / fingerprinting (as in plant pathogens or bacteria), community profiling where sequence divergence is high.46
37. Suicide PCR
Suicide PCR is a PCR approach that is used, especially in paleogenetics or ancient DNA studies, in which primer combinations are used only once in a laboratory setting, so that contamination from prior amplifications is minimized.
With primers unused in any positive-control reactions, and targeting a genomic region never previously amplified in that lab, which ensures that no carry-over contaminant from prior PCRs can generate a false positive.
In samples where DNA is degraded or ancient, and where target DNA abundance is very low, suicide PCR is advantageous because the risk of amplifying contaminant DNA (ubiquitous in labs) is reduced.
Stringent laboratory practices are required; reagents, pipettes, surfaces must be clean, and a workflow ensuring that primers truly have never been used in any detectable reaction is enforced.
Amplification conditions are standard PCR but validation of product (e.g. by sequencing) is usually done to confirm authenticity, since even with precautions low level false positives may occur.
Sensitivity is lowered relative to PCR with positive controls, because no positive control is used (to avoid contaminant amplification), which means risk of false negatives is increased.
Specificity is very high, because only amplification from truly present target DNA happens (if everything is clean), which is especially needed for claims about ancient pathogens or low copy number sequences.
Limitations / considerations are present: missing detection (false negative) if target is too degraded, DNA damage may hamper primer binding; no positive control means less ability to verify reaction worked; demands high lab discipline and documentation.47
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.