Microscope Slides are thin, flat pieces of glass or plastic, it is used to hold specimens for microscopic observation. This allows light or electrons to pass through, then the specimen can be seen clearly under the microscope.
Purpose of Slides is to support and protect the specimen, it keeps it flat and in place. This ensures clear and focused viewing, then prevents damage to both the specimen and the microscope lens.
Types of Slides include glass, plastic, and frosted slides. Glass slides are reusable and good for staining, plastic slides are disposable, then frosted slides are labeled easily to avoid confusion.
Preparation of Slides involves placing the specimen on the slide, then covering it with a cover slip. This prevents drying, protects the lens, and keeps the specimen in position.13
Uses of Slides are in biology, medicine, and research, it allows observation of cells, tissues, and microorganisms. This is important for diagnosis, study, and teaching, then helps in learning and experiments.
Special Slides may be coated with adhesives or chemicals, it helps tissues stick and enhances staining. This improves visibility of specific structures, then makes microscopic study easier and more accurate.9
What are microscope slides used for?
- Microscope Slides are used to hold specimens for microscopic observation, it keeps the sample flat and stable. This allows light or electrons to pass through, then the structures inside can be seen clearly.
- Observation of Cells and Tissues – slides are used to study plant and animal cells, it helps in identifying cell shapes, structures, and arrangements. Then it is essential for learning biology and medical research.
- Microorganism Study – they allow viewing bacteria, fungi, protozoa, and algae, it is used in microbiology labs for research and diagnosis. Then scientists can track growth, behavior, and response to treatments.
- Medical Diagnosis – slides are used for blood smears, tissue samples, and other clinical specimens, it helps detect infections, diseases, or abnormalities. Then doctors and lab technicians rely on slides for accurate results.
- Research and Teaching – in labs and classrooms, slides help demonstrate biological processes, it allows experiments, staining techniques, and observation of reactions. Then it improves understanding of complex microscopic structures.
- Industrial and Environmental Use – slides are also used to study water quality, soil microbes, or industrial materials, it helps monitor contamination and ecological changes. Then it supports environmental research and safety studies.2
What are the different types of microscope slides?
Here are the different types of microscope slides;
- Glass Slides – these are most common, made of clear glass, it is reusable after cleaning. Then it is ideal for staining and mounting specimens, widely used in labs, schools, research.
- Plastic Slides – lighter than glass, less breakable, it is often disposable. This makes them good for teaching labs or fieldwork, but they scratch easily and may not handle staining well.
- Frosted Slides – have frosted end for labeling, it helps identify samples quickly. Then it is useful when many slides are handled, available in glass or plastic types.
- Concave (Well) Slides – these have a small depression in center, it holds liquid specimens, useful for live organisms. Then it allows larger samples to be studied without spilling, used in microbiology.
- Charged Slides – specially treated with positive charge, it helps tissues stick better. Then it is used for delicate specimens or staining processes, reduces sample loss.
- Cover Glass – thin glass placed over specimens, it flattens samples and protects microscope lens, then ensures clear viewing.
- Specialty Slides – slides with reaction wells, coatings, or other features, it is made for specific experiments. Then used in diagnostic tests, research, or industrial applications.3
How do I prepare a microscope slide?
- Step 1 – Clean the Slide – first, take a clean microscope slide, it should be free of dust, grease, or fingerprints. Then this prevents smudges and ensures the specimen can be seen clearly under microscope.
- Step 2 – Place the Specimen – put the sample in the center of the slide, it can be a thin tissue, cell smear, or a drop of liquid. Then arrange it flat so it spreads evenly and doesn’t fold or clump.
- Step 3 – Add Liquid or Stain – for wet mounts, add a drop of water, saline, or stain, it helps hold the specimen in place and improves contrast. Then be careful to avoid air bubbles, they block viewing.
- Step 4 – Cover the Specimen – gently place a cover slip over the sample, it protects the specimen and keeps it flat. Then press lightly to remove extra liquid, it ensures clear observation and prevents slipping.10
- Step 5 – Label the Slide – write the specimen name on the frosted end if present, it helps identify samples easily. Then this is useful for experiments, teaching, or long-term storage.
- Step 6 – Observe Under Microscope – place the prepared slide on the microscope stage, it is ready for viewing. Then adjust focus, light, and magnification, it ensures a clear and detailed image of the specimen.
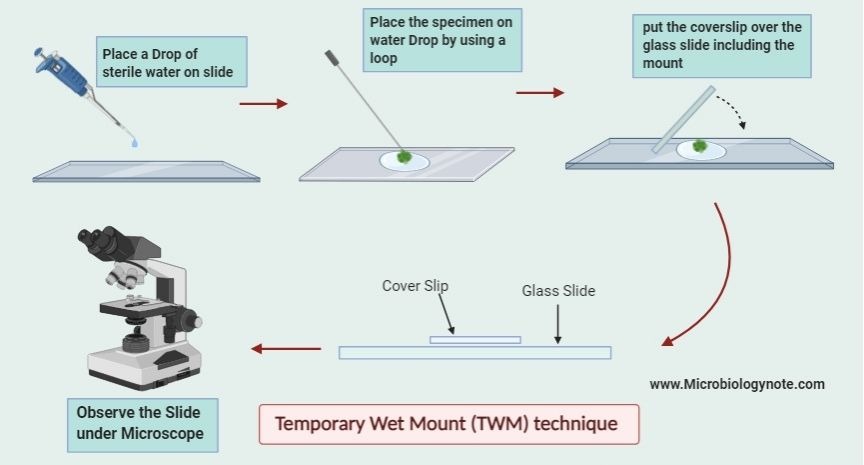
How to Prepare a microscopic Slide for Wet Mount (or Temporary Mount) technique?
- Step 1 – Clean the Slide – take a clean glass slide, it must be free from dust, grease or fingerprints. Then this ensures clear observation and avoids contamination.
- Step 2 – Place the Specimen – put a small piece of tissue, cell smear, or drop of liquid in the center of the slide, it should be thin enough to allow light to pass. Then spread gently to avoid clumping.
- Step 3 – Add a Drop of Water or Stain – place a drop of water, saline, or stain on the specimen, it keeps it moist and improves visibility. Then avoid air bubbles as they block light and distort the view.
- Step 4 – Place the Cover Slip – hold a cover slip at an angle and gently lower it over the specimen, it prevents trapping air bubbles. Then press lightly to remove excess liquid and keep the sample flat.
- Step 5 – Remove Excess Liquid – if water or stain spreads outside, use blotting paper, it absorbs extra fluid. Then this prevents slipping and keeps the slide neat.
- Step 6 – Observe Under Microscope – place the prepared slide on the microscope stage, it is now ready for observation. Then adjust focus, illumination, and magnification for clear viewing.
- Step 7 – Temporary Storage or Disposal – wet mount slides are temporary, it should be cleaned after use. Then do not store long-term as specimens may dry or degrade.1
Tips:
- Keep the sample thin for light to pass through.
- Avoid overfilling with liquid to prevent spills.
- Use stain sparingly (if needed) to enhance visibility.
How to Prepare a microscopic Slide for Dry mount technique?
- Step 1 – Clean the Slide – first, take a clean glass slide, it should be free from dust, grease or fingerprints. Then this ensures the specimen can be seen clearly, it prevents contamination.
- Step 2 – Select the Specimen – choose a thin, small, dry specimen like a leaf, hair, or insect part, it should be flat enough to fit under the cover slip. Then trim if needed to avoid overlapping or thick spots.
- Step 3 – Place the Specimen – gently place the specimen in the center of the slide, it must lie flat and stable. Then adjust position carefully so all parts are visible under the microscope.
- Step 4 – Cover the Specimen – place a cover slip over the specimen, it protects it and keeps it flat. Then press lightly to prevent slipping, but avoid crushing delicate parts.14
- Step 5 – Label the Slide – write the specimen name on the frosted end if available, it helps identify the slide later. Then this is useful for experiments, teaching, or storage.
- Step 6 – Observe Under Microscope – place the prepared slide on the microscope stage, it is ready for viewing. Then adjust focus, illumination, and magnification for a clear image.
Tips:
- Keep the specimen thin and flat for clear viewing.
- Skip the coverslip for thick or 3D samples (e.g., insects).
- Avoid overloading with adhesive to prevent distortion.
Smear Mount Preparation
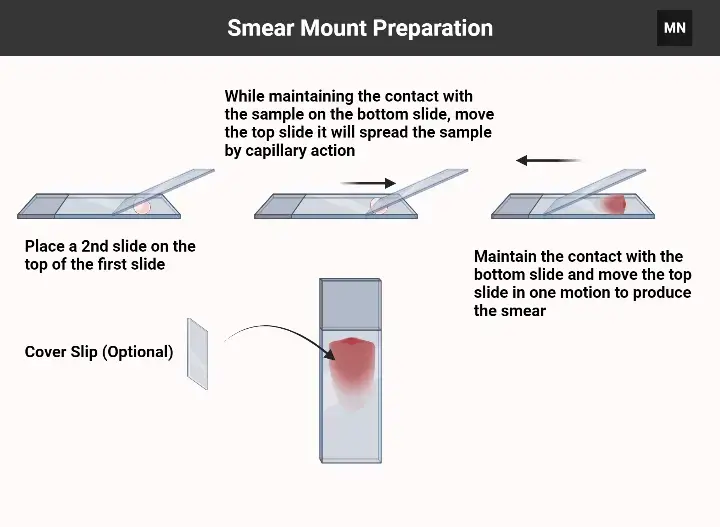
- Gather materials: Clean microscope slides, inoculating loop, specimen (liquid culture or colony), water/saline (if needed), Bunsen burner (for heat fixation), stains, and bibulous paper.
- Prepare the slide – Clean the slide with ethanol or soapy water, then dry with a lint-free cloth.
- Apply the specimen:
- Liquid culture: Place a small drop on the slide using a sterilized loop.
- Solid culture: Mix a colony with a drop of water/saline on the slide to create a suspension.
- Spread the sample– Use the loop or edge of another slide to thinly spread the sample into a film (~1 cm diameter).
- Air-dry– Let the slide dry completely at room temperature (do not heat).
- Heat-fix. – Pass the slide (sample-side up) through a flame 2–3 times to adhere cells to the slide. Avoid overheating.
- Stain (optional) – Apply stain (e.g., Gram stain, methylene blue) for the recommended time.
- Rinse gently– Tilt the slide and rinse with water to remove excess stain.
- Blot dry– Gently pat the slide with bibulous paper or let it air-dry.
- Examine under a microscope– Place the slide on the stage, add immersion oil (if using oil immersion lens), and view.415

Staining a Sample in Microscopic Slide
- Prepare the smear: Ensure the slide is air-dried and heat-fixed (as per smear preparation steps).
- Gather materials: Stains (e.g., crystal violet, safranin, methylene blue), staining rack, distilled water, dropper/bottle, decolorizer (e.g., ethanol/acetone for Gram staining), bibulous paper, gloves.
- Apply primary stain– Flood the smear with the first stain (e.g., crystal violet for Gram staining) and let sit for 1–2 minutes.
- Rinse gently– Tilt the slide and rinse with distilled water to remove excess stain.
- Apply mordant (if required)– For some stains (e.g., Gram’s iodine in Gram staining), flood the slide and wait 1 minute, then rinse.
- Decolorize (if required)– Add decolorizer (e.g., ethanol) drop by drop until runoff is clear (~5–10 seconds). Rinse immediately with water.
- Apply counterstain – Flood with a contrasting stain (e.g., safranin for Gram staining) for 1–2 minutes, then rinse.
- Blot dry – Gently pat the slide with bibulous paper or air-dry.
- Examine under a microscope– Place the slide on the stage, add immersion oil (for oil immersion lens), and observe.1116
Note:
- Steps vary by staining method (e.g., Gram stain vs. acid-fast stain).
- Always handle stains with gloves and dispose of chemical waste properly.
How to study glass microscope slides?
- Step 1 – Place the Slide – first, put the prepared slide on the microscope stage, it should be centered and held by stage clips. Then this keeps it steady, so the specimen doesn’t move while observing.
- Step 2 – Select Low Power Objective – start with the lowest-power lens, usually 4x or 10x, it gives a wide view of the specimen. Then it helps locate the part you want to see clearly.
- Step 3 – Adjust Coarse Focus – look through the eyepiece and use the coarse focus knob, it brings the specimen into rough focus. Then be careful not to touch the slide with the lens, it may damage both.
- Step 4 – Fine Focus – after rough focus, use the fine focus knob, it sharpens the image and shows details. Then adjust slowly until the structures are clear, it is important for high accuracy.
- Step 5 – Increase Magnification – rotate to a higher-power objective lens like 40x or 100x, it zooms in on details. Then always use fine focus again, it prevents blurring or damage.
- Step 6 – Adjust Lighting – change the diaphragm or condenser, it enhances contrast and visibility. Then it helps see internal structures, especially in transparent specimens.
- Step 7 – Observe and Note – carefully examine the specimen, it may include cells, tissues, or microorganisms. Then make sketches or notes, it helps in recording observations and study.
- Step 8 – Clean and Store – after use, turn off light, remove the slide, and clean lenses with lens paper. Then store the microscope safely, it keeps the equipment ready for next use.12
What are Prepared Slides?
Prepared Slides are microscope slides that already have specimens mounted on them, it may be stained or unstained, then they are ready to observe immediately without any extra preparation. They save time in labs, it allows students and researchers to focus on observation rather than making slides.
Specimens on Prepared Slides include plant tissues, animal tissues, blood smears, microorganisms, it often uses stains to highlight structures. Then this helps in easier identification of cells, tissues, or microbial forms under the microscope.
Advantages of prepared slides are they are ready to use, it reduces errors during preparation, and preserves delicate or rare specimens for long-term study. Then it allows repeated observation without damaging the sample.
Usage involves placing the slide on the microscope stage, it can be viewed directly under appropriate magnification. Then adjust focus and lighting carefully, it ensures a clear and detailed image of the specimen.
How to Make a Blood Smear Slide
- Step 1 – Collect the Blood Sample – first, take a clean lancet or needle to obtain a small drop of blood, it is usually from a fingertip or vein. Then handle carefully to avoid contamination or injury.
- Step 2 – Prepare the Slide – take a clean glass slide, it should be free from dust or grease. Then place one drop of blood near one end of the slide.
- Step 3 – Spread the Blood – use another clean slide held at 30–45° angle, touch the drop and pull it back to spread the blood. Then push the slide forward smoothly to make a thin, even smear.
- Step 4 – Air Dry the Smear – allow the smear to air dry completely, it should not be blown or wiped as this can damage cells. Then it becomes ready for staining.
- Step 5 – Fixing (Optional) – some procedures require fixing with methanol, it preserves cell structure. Then this prevents washing off during staining.
- Step 6 – Staining – apply a suitable stain like Giemsa or Wright’s stain, it helps differentiate blood cells. Then wait for appropriate time and rinse gently with water.
- Step 7 – Observe Under Microscope – place the stained slide on microscope stage, it is ready for viewing. Then use appropriate magnification and adjust focus for clear observation of blood cells.5
Pro tips:
- If the smear looks streaky, you pushed the spreader too fast or at the wrong angle.
- Wear gloves to avoid mixing your skin oils with the sample.
- Practice makes perfect—mess up a few slides? Totally normal!
What are the materials needed for preparing a microscope slide?
- Microscope Slides – standard glass slides, about 75mm × 25mm, 1mm thick, clear and flat.
- Cover Slips – thin, square or round glass pieces, 18mm × 18mm or 22mm × 22mm, placed over specimens to protect and flatten them.
- Tweezers – fine-tipped, stainless steel or plastic, used to handle delicate specimens without contamination.
- Dropper or Pipette – for placing liquids like water or stains onto slides, ensuring controlled application
- Stains – dyes like iodine, methylene blue, or eosin, used to enhance contrast and highlight cellular structures.
- Forceps – used to place or remove specimens from slides, especially when dealing with small or fragile samples.
- Scalpel or Razor Blade – for cutting thin slices of specimens, particularly in preparing tissue sections.
- Lens Paper or Cloth – for cleaning slides and cover slips to remove dust, fingerprints, or stains before use.
- Staining Dishes or Petri Dishes – used to hold and immerse slides in staining solutions, ensuring even application.
- Distilled Water – used to rinse slides and dilute stains, preventing contamination from tap water.
- Paper Towels or Blotting Paper – for drying slides after rinsing or to remove excess liquid.
- Microscope – used to observe the prepared slides, typically with multiple objective lenses for varying magnification.
- Slide Storage Box – for organizing and safely storing prepared slides to prevent damage.
- Labeling Materials – such as waterproof pens or stickers, for marking slides with specimen information.
- Timer – to monitor staining times, ensuring consistent results.
- Hot Plate or Hair Dryer – used to dry slides quickly after staining or to fix specimens.
- Sealant (Optional) – like clear nail polish, to seal the edges of cover slips, preventing specimen dehydration
- Specimen Collection Tools – such as cotton swabs or spatulas, for collecting samples from various sources.
- Safety Equipment – like gloves and goggles, to protect against stains and sharp instruments.
- Disposal Containers – for safely discarding used materials like broken slides or used staining solutions.
- Instructional Materials – such as guides or videos, to assist in proper slide preparation techniques.7
What is the purpose of using a cover slip?
- Protection of Specimen – a cover slip protects the specimen on the slide from dust, dirt, and physical damage, it keeps it flat and intact. Then this prevents the sample from drying out or getting crushed during observation.
- Improved Viewing – it helps in spreading the specimen evenly, it reduces thickness, then allows light to pass uniformly for clearer microscopic images.
- Lens Protection – the cover slip prevents the microscope’s objective lens from touching the specimen, it avoids smudges and scratches. Then this prolongs the life of expensive lenses.
- Minimizes Air Bubbles – when placed carefully, it reduces air pockets that can distort the view, it ensures the specimen can be seen clearly. Then it also stabilizes wet mount slides for better observation.
- Facilitates Staining – in stained slides, it holds the stain in place, it prevents evaporation and keeps the specimen visible. Then this improves contrast and highlights structures effectively.
How do I clean microscope slides?
- Step 1 – Remove Excess Specimen – first, wipe off any remaining specimen or stain from the slide, it can be done gently with a paper towel or soft cloth. Then this prevents scratching and makes cleaning easier.
- Step 2 – Rinse with Water – rinse the slide under running tap water or distilled water, it removes loose debris and residues. Then avoid using very hot water as it may crack the glass.
- Step 3 – Use Cleaning Solution – apply a mild detergent or 70% ethanol, it helps remove grease, oil, or stubborn stains. Then gently scrub with a soft brush or lint-free cloth if needed.
- Step 4 – Rinse Again – rinse the slide thoroughly with distilled water, it ensures no detergent or chemical residue remains. Then leftover residue can interfere with microscopy.
- Step 5 – Dry the Slide – wipe the slide gently with lens paper or air dry, it prevents scratches. Then store the cleaned slide in a slide box to protect it from dust.
- Step 6 – Handle with Care – always hold slides by the edges, it avoids fingerprints on the surface. Then this keeps slides clear and ready for use.17
What are the common mistakes to avoid while preparing microscope slides?
- Using Dirty Slides or Cover Slips – always use clean slides and cover slips, it prevents dust, grease, or fingerprints from interfering with observation. Then dirty slides can distort images or contaminate specimens.
- Too Thick or Uneven Specimens – placing thick or uneven samples, it makes focusing difficult and blocks light. Then thin, flat specimens allow clearer viewing and proper light passage.
- Air Bubbles – trapping air under the cover slip, it can distort the view of the specimen. Then place the cover slip gently at an angle to reduce bubbles.
- Excess Liquid – adding too much water or stain, it can overflow and make the slide messy. Then blot extra liquid carefully without damaging the specimen.
- Improper Staining – using too strong or weak stain, it can hide structures or fail to show details. Then follow recommended staining time and concentration.
- Touching the Specimen Surface – handling the specimen directly with fingers, it can introduce oils and contamination. Then always use tweezers, pipettes, or forceps.
- Ignoring Safety Precautions – not wearing gloves or goggles, it risks contact with stains or sharp instruments. Then always follow lab safety rules.
- Rushing the Process – preparing slides too quickly, it may lead to uneven spreading, broken cover slips, or damaged specimens. Then work carefully and methodically for best results.
How do I store prepared microscope slides?
- Clean Before Storing – first, make sure slides are clean and dry, it prevents dust, stains, or mold growth. Then remove any excess stain or liquid before storage.
- Use Slide Boxes – store slides in dedicated slide boxes or racks, it keeps them organized and prevents breaking. Then boxes with compartments help separate delicate or rare specimens.
- Label Properly – write the specimen name and date on the frosted end or label, it makes identification easy. Then proper labeling avoids mix-ups when many slides are stored together.
- Keep in Dry Place – store slides in a cool, dry environment, it prevents moisture damage or fungal growth. Then avoid direct sunlight or humid areas which can deteriorate specimens.
- Handle Carefully – always pick slides by edges, it avoids fingerprints and scratches. Then this keeps them clean and ready for future use.
- Avoid Overcrowding – do not stack slides directly on top of each other, it can break or scratch them. Then use boxes or racks designed for multiple slides safely.8
What are the safety precautions to take while preparing microscope slides?
Safety concerns in microscope slide preparation revolve around activities involving materials and chemicals. –
- Wear Gloves and Goggles – always use protective gloves and goggles, it prevents contact with stains, chemicals, or biological specimens. Then it reduces the risk of irritation or injury.
- Handle Slides Carefully – hold slides by the edges, it avoids fingerprints and accidental cuts. Then always be cautious with glass slides and cover slips as they can break easily.
- Use Sharp Instruments Safely – when using scalpels, razor blades, or needles, it is important to cut away from yourself. Then dispose of blades safely in a sharps container to prevent accidents.
- Avoid Direct Contact with Stains – stains and chemicals can be toxic or irritating, it is important not to touch them directly. Then use droppers or pipettes and wash hands after handling.
- Work on a Stable Surface – prepare slides on a flat, stable surface, it prevents spills or slides from falling. Then this reduces the risk of breakage or contamination.
- Dispose of Waste Properly – used slides, broken glass, or excess stains must go into proper disposal containers, it prevents injury and contamination. Then follow lab safety protocols for hazardous materials.
- Keep Area Clean – maintain a clean workspace, it prevents contamination and accidents. Then always clean up spills immediately and store equipment safely.
How do I dispose of used microscope slides safely?
- Handle with Care – always pick up used slides by the edges, it prevents cuts from broken glass. Then never touch the specimen area directly to avoid contamination.
- Separate Broken Slides – if a slide is cracked or shattered, place it in a designated sharps or broken glass container, it keeps others safe. Then do not throw broken slides in regular trash.
- Use Proper Disposal Containers – place used slides in puncture-resistant containers or sharps bins, it prevents injuries and contamination. Then clearly label the container as “biohazard” if it contains biological specimens.
- Decontaminate if Necessary – for slides with infectious material, immerse in disinfectant like 10% bleach, it kills microbes before disposal. Then follow lab protocols for biohazard waste.
- Do Not Reuse Contaminated Slides – avoid reusing slides that had infectious samples, it prevents accidental exposure. Then only clean and reuse slides that had non-hazardous specimens.
- Follow Laboratory Guidelines – always adhere to institutional or local regulations for disposal, it ensures safety and legal compliance. Then proper disposal protects both people and environment.6
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.