What is Polyacrylamide Gel Electrophoresis (PAGE)?
- PAGE can be defined as a laboratory method by which charged biomolecules (proteins or nucleic acids) are separated by applying an electric field through a gel made of polyacrylamide.
- The principle is that molecules migrate through a porous matrix and are slowed by size/shape, so smaller or more mobile move faster, larger slower.
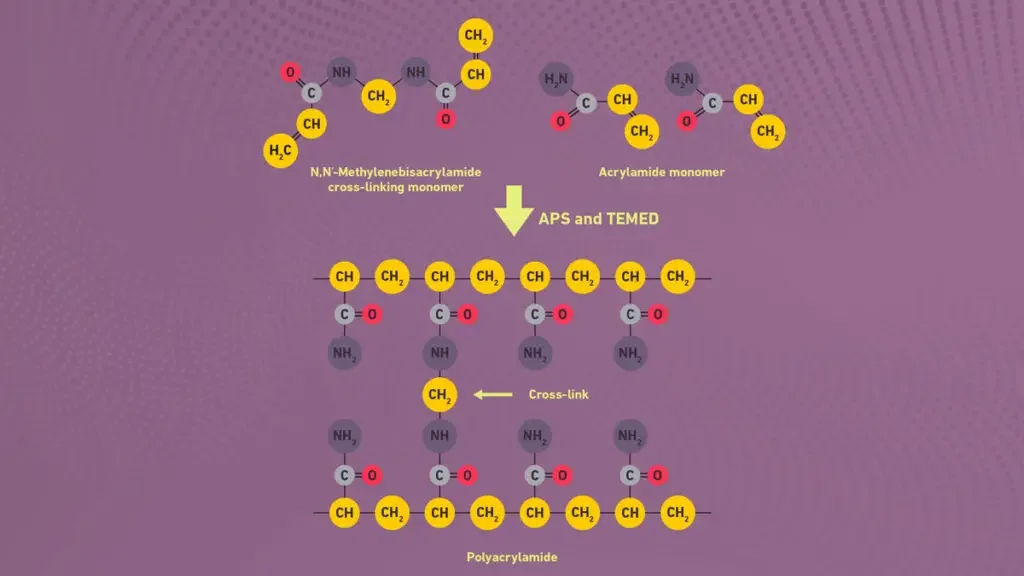
- In PAGE, a gel matrix of acrylamide and a cross-linker (e.g., bis-acrylamide) is polymerized (often using ammonium persulfate (APS) and TEMED) to form a network of pores.
- The pore size is controlled by the concentrations of acrylamide and bis-acrylamide, so that the gel behaves like a molecular sieve.
- A sample containing proteins (or nucleic acids) is loaded in wells at one end of the gel; an electric current is applied, the molecules migrate toward the electrode of opposite charge.
- Under “denaturing” conditions (for proteins) a detergent like SDS (sodium dodecyl sulfate) is used to give the proteins a uniform negative charge and to remove shape/charge differences so separation is mostly by size (molecular weight) rather than shape/charge.
- Under “native” conditions (without denaturing) the molecules keep their higher-order structure and separation depends on size, shape and charge.
- After the run the gel is stained (for example with Coomassie blue for proteins) and the separated bands (zones) are visualised and can be analysed.
- PAGE is extremely important in biochemistry, molecular biology, genetics because it allows the separation, identification, estimation of size/purity of biomolecules (especially proteins) in mixture.
- It allows researchers to monitor protein expression, check for presence/absence of proteins, assess purity, observe sub-units of complexes.
- PAGE’s ability to control resolution (via gel % and matrix) makes it powerful for analysing molecules of different sizes with high accuracy.
- The technique underlies many downstream analyses (western blotting, proteomics, protein characterisation) and is a work-horse method in many labs.
- Its versatility (native vs denaturing, gradient gels, altering pore sizes) gives flexibility for many experimental designs.
- The use of synthetic polyacrylamide gel as a support medium was introduced around 1959: the gel medium allowed better control of pore size and molecular sieving effects.
- Earlier forms of electrophoresis (moving boundary, paper electrophoresis) were used in the 1930s-1950s but lacked the resolution and reproducibility of gels
- The term “zone electrophoresis” appeared around 1950 (by Arne Tiselius) when the shift from moving boundary to zone gels occurred.
- Polyacrylamide gel electrophoresis rapidly became standard because the synthetic gel is stable, reproducible, transparent, capable of high resolution.
- Over time, refinements (stacking gels, discontinuous gel systems, SDS denaturation) improved resolution and utility.
Definition of Polyacrylamide Gel Electrophoresis (PAGE)
Polyacrylamide Gel Electrophoresis (PAGE) is a technique used to separate and analyze proteins or nucleic acids based on their mobility in a porous polyacrylamide gel matrix under the influence of an electric field.
Types of Polyacrylamide Gel Electrophoresis (PAGE)
Native PAGE – Proteins (or nucleic-acids) are separated under their natural (non-denatured) conditions, so the shape, charge and size all affect mobility, rather than just size.
Denaturing PAGE – The molecules are unfolded / linearised (for proteins by detergent like Sodium dodecyl sulfate (SDS) and sometimes reducing agents) so that separation is mostly by size (mass) rather than shape/charge.
SDS-PAGE – A specific form of denaturing PAGE for proteins, where SDS coats the polypeptides giving roughly uniform charge:mass ratio, and then they are separated by molecular weight (smaller travel faster).
Reducing SDS-PAGE – Similar to SDS-PAGE but with a reducing agent (eg. DTT or β-mercaptoethanol) added to break disulfide bonds, so subunits of multi-unit proteins separate.
Gradient-PAGE / 2D-PAGE – A gradient gel has varying acrylamide concentration (eg from low % to higher %) so a broad size-range of molecules can be resolved, or in 2-D PAGE the first dimension is by one property (like isoelectric point) and second dimension by molecular weight.
Other specialised PAGE types – There are special versions like native continuous PAGE systems, or variants for nucleic-acids (denaturing urea-PAGE) and gel systems for high resolution of small fragments.
Principle of Polyacrylamide Gel Electrophoresis (PAGE)
- Polyacrylamide Gel Electrophoresis (PAGE) refers as a biochemical technique used for separation of proteins, nucleic acids etc. according to their charge/size.
- In this method, separation is done by migration of charged molecules by a gel matrix (polyacrylamide).
- The principle based on the movement of charged molecules in an electric field, where smaller molecules move faster than larger ones.
- The gel act as a molecular sieve, controlling the rate of migration by pore size.
- The polyacrylamide gel formed by polymerization of acrylamide and bisacrylamide (cross-linker) in presence of free radical initiator like ammonium persulfate (APS) and TEMED.
- By the concentration of acrylamide/bisacrylamide, the pore size can vary which affect resolution of protein bands.
- The proteins usually carry different net charges depend on their amino acid composition and pH of buffer used.
- When the electric current is applied, molecules are moved towards the electrode of opposite charge, that is negatively charged proteins go to the anode.
- In PAGE, buffer maintain the pH and ionic strength, prevent overheating and diffusion of bands.
- Migration rate depends on several factors – molecule size, shape, net charge, and gel concentration etc.
- Two main types of PAGE are used, Native PAGE (without SDS) and SDS-PAGE (with sodium dodecyl sulfate).
- In native PAGE, proteins retain their natural structure and charge so they separate by charge/mass ratio.
- In SDS-PAGE, all proteins are denatured and coated with SDS giving uniform negative charge, hence separation only by size.
- The gel is generally placed vertically between glass plates and submerged by running buffer, electric current is then applied from top to bottom.
- The movement of molecules continues until the smallest fragments reach the bottom, forming visible or stained bands.
- The migration pattern gives information about purity and approximate molecular weight of proteins.
- After electrophoresis, visualization done by staining methods like Coomassie Brilliant Blue or Silver stain.
- It is important that polymerization of gel mixture be complete to avoid uneven band pattern.
- The PAGE principle mainly rely on electrophoretic mobility μ which depend on charge to frictional ratio (μ=q/f).
- This method provide a sturdy and hardy analytical approach for biochemical and molecular biology studies, though not without limitations etc.
Requirements for Polyacrylamide Gel Electrophoresis (PAGE)
To perform Polyacrylamide Gel Electrophoresis (PAGE), several requirements and equipment are necessary. These include:
- Power supply and electrophoresis unit are required for generating electric field that drive movement of charged molecules by the gel matrix.
- Polyacrylamide gel act as supporting medium, prepared by polymerization of acrylamide and bisacrylamide in specific ratio for desired pore size.
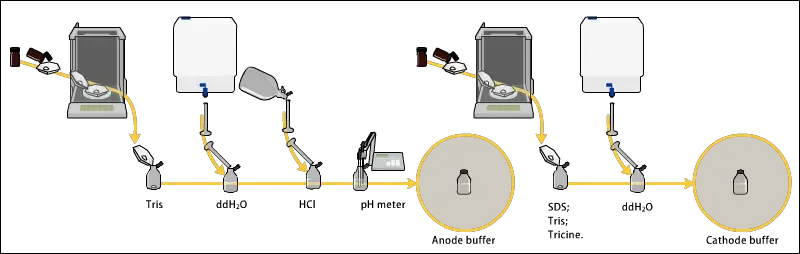
- Buffer solutions like Tris-glycine or Tris-HCl are used for maintaining constant pH and ionic strength during run.
- A gel casting apparatus (plates, spacers, combs) needed for forming gel slab of uniform thickness and wells for loading samples.
- Sample loading buffer (contain glycerol, tracking dye, SDS sometimes) is used for giving density and visibility to the samples during electrophoresis.
- SDS (sodium dodecyl sulfate) reagent is needed when performing SDS–PAGE to denature proteins and provide uniform negative charge.
- Ammonium persulfate (APS) and TEMED are used as catalyst system for initiating free radical polymerization of acrylamide monomers.
- Protein samples or nucleic acid samples are the analytes to be separated by the electrophoretic process.
- A staining solution like Coomassie Brilliant Blue or Silver stain used after run to visualize separated bands clearly.
- Distilled or deionized water is used in preparing all solutions to avoid impurities which affect migration.
- Pipettes and micropipette tips are required for accurate measurement and loading of small sample volumes (usually µL range).
- Glass plates are needed for sandwiching the gel, they must be clean and dry for uniform polymerization.
- Gel tank with electrodes is required for running current by gel, usually one electrode at top and other at bottom.
- pH meter sometimes used to check pH of buffers since stability of gel depend on correct pH range.
- Protein markers/standards used for estimating molecular weight of unknown protein bands by comparison.
- During PAGE, cooling system or ice bath may be required to prevail overheating caused by electric current.
- Destaining solution required after staining step to remove background color and make bands distinct.
- Protective equipment like gloves, goggles, and lab coat must be used since acrylamide is neurotoxic in unpolymerized form.
- For data analysis, gel documentation system or simple light box can used for photographing the stained gel.
- These requirements together ensure proper functioning, reliability, and safety of Polyacrylamide Gel Electrophoresis (PAGE) technique etc.
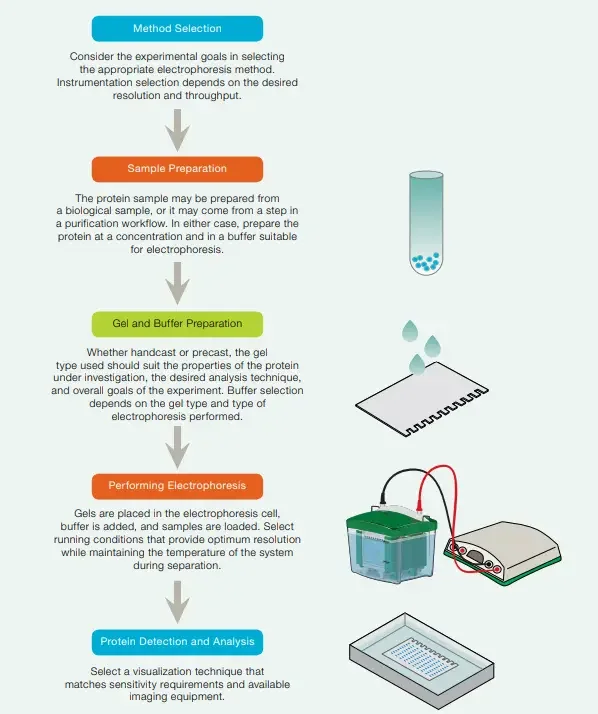
Steps Involved in Polyacrylamide Gel Electrophoresis (PAGE)
1. Sample preparation
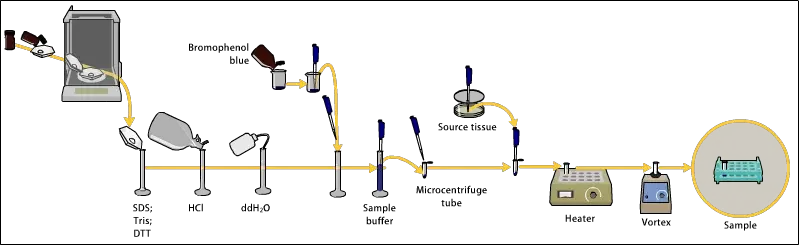
- Collection of sample is done from protein or nucleic acid sources which needed to be separated by PAGE technique.
- The sample concentration is adjusted to proper range so that distinct and visible bands are obtained after electrophoresis.
- Buffer addition is performed using sample buffer (contain Tris-HCl, SDS, glycerol, bromophenol blue, etc.) to maintain pH and density.
- Denaturation of proteins is usually carried out by heating the sample at 95°C for 3–5 min, that help in breaking secondary and tertiary structures.
- The presence of SDS (sodium dodecyl sulfate) in buffer provides uniform negative charge to all protein molecules.
- Sometimes β-mercaptoethanol (β-ME) or DTT (dithiothreitol) is added for reducing disulfide bonds in protein structure.
- Mixing is done properly so that buffer and protein sample are completely homogenized without bubbles.
- The sample volume should be small, usually 10–20 µL depending on well size of gel.
- After denaturation, the sample is cooled on ice for few minutes to prevail re-aggregation.
- The loading dye (tracking dye) like bromophenol blue help in monitoring migration of sample during electrophoresis run.
- Before loading, the sample may be centrifuged shortly (for 30 sec) to remove insoluble particles or bubbles.
- The prepared samples are carefully loaded into wells of polyacrylamide gel using micropipette.
- Molecular weight markers are also loaded in one separate well for comparison of protein sizes.
- Sometimes, native PAGE performed without SDS and reducing agents, in which protein kept in native folded form.
- The prepared samples must be used freshly or stored at -20°C if delay occur before electrophoresis.
- All these steps ensure that protein molecules migrate according to their molecular weight/charge properly during PAGE run.
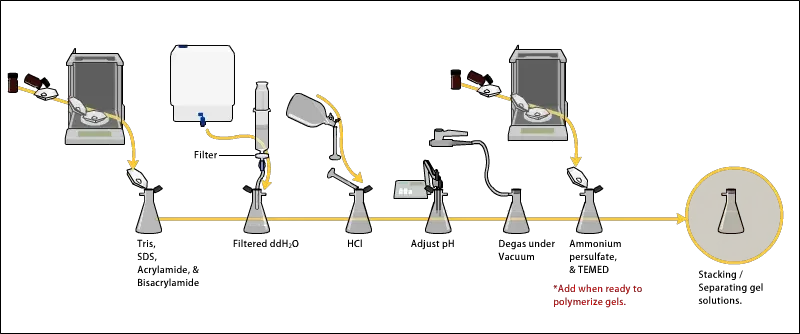
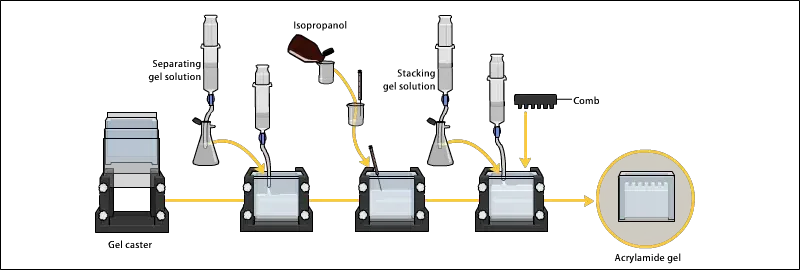
2. Preparing acrylamide gels
- The gel solution is prepared by mixing acrylamide and bis-acrylamide solution in specific ratio which decide pore size of gel, usually 30:1 ratio used for protein separation.
- In the mixture, Tris-HCl buffer is added for maintaining pH, mostly 8.8 for resolving gel and 6.8 for stacking gel.
- Then distilled water is added to adjust the final volume of solution according to the gel size required.
- Before polymerization, SDS (sodium dodecyl sulfate) is often added (0.1%) to denature proteins if SDS–PAGE is performed.
- The solution must be de-gassed by mild vacuum or nitrogen bubbling, this help to remove oxygen which inhibit polymerization reaction.
- The polymerization process is initiated by adding ammonium persulfate (APS) and TEMED (tetramethylethylenediamine), these create free radicals to start chain reaction.
- The resolving gel is poured first between glass plates up to required height (about 3/4th of plate) and then covered with layer of water or butanol to level the surface and prevail oxygen contact.
- After polymerization (takes about 20–30 min), the water/butanol is removed gently by pipette and surface is washed.
- Then the stacking gel solution is prepared separately using lower acrylamide concentration (around 4%), lower pH buffer (6.8), and same APS/TEMED initiator system.
- The stacking gel is poured over polymerized resolving gel, and a comb is inserted immediately to form wells for sample loading.
- After complete polymerization (10–20 min), the comb is carefully removed and wells are rinsed with running buffer to remove unpolymerized acrylamide residues.
- The prepared gel is now ready for assembly in electrophoresis apparatus and can be used instantly or stored in moist condition for short time.
- The accuracy of polymerization, ratio of acrylamide/bis-acrylamide, and pH adjustment strongly affect the quality of bands during electrophoresis etc.
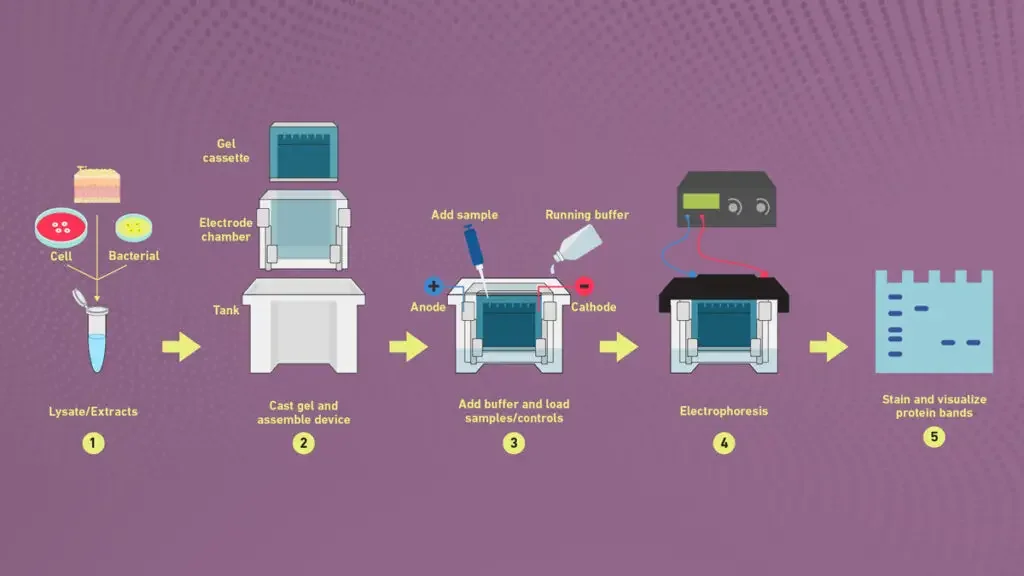
3. Electrophoresis
- The gel assembly is placed properly in the electrophoresis apparatus and tightened to avoid any leakage of running buffer.
- Running buffer (like Tris-Glycine or Tris-Tricine) is poured into both upper and lower tank till the electrodes are fully immersed.
- The wells of gel are rinsed gently by buffer to remove unpolymerized residues and bubbles that may disturb sample loading.
- Prepared protein samples are then loaded carefully into wells by micropipette along with molecular weight markers in one well.
- The samples should settle properly in wells, and no overflow or mixing between adjacent wells should occur.
- The power supply is connected ensuring correct polarity, since negatively charged proteins/SDS complexes migrate toward the anode (+).
- The voltage is applied usually between 80–120 V for stacking part and then increased to 120–200 V during resolving stage depending on gel type.
- During run, the bromophenol blue tracking dye is monitored to follow the progress of electrophoresis, which moves ahead of protein bands.
- The electrophoresis continues until dye front reaches near the bottom of the gel, normally it takes about 1–2 hours.
- Overheating should be avoided, so apparatus sometimes kept in cooling condition or with circulating buffer.
- After completion of run, the power is turned off, and gel plates are carefully dismantled with spatula or gel knife.
- The gel is separated from glass plate gently and transferred to staining tray without tearing.
- The staining step follows immediately for visualization of separated protein bands, often using Coomassie Brilliant Blue or Silver stain.
- Once bands are fixed and visible, the gel can be photographed or analyzed for molecular weight estimation etc.
4. Detection
- After electrophoresis, the gel is gently removed from the glass plates and it is placed in a tray for staining process.
- The Coomassie Brilliant Blue (CBB) stain is usually used for detection of proteins because it binds with amino acid residues mainly arginine and aromatic ones.
- The gel is immersed in the staining solution which contain CBB R-250 dissolved in methanol:acetic acid:water mixture (5:1:4 ratio usually used).
- The staining is done by slow agitation at room temperature for 1–2 hr or till blue color appear clearly in protein bands.
- Sometimes heating is also used for quick staining but too high temp can deform the gel or diffuse bands.
- After that, the gel is transferred to destaining solution (methanol/acetic acid/water again but in different ratio) to remove excess dye background.
- The destaining process is carried out by several changes of solution until clear transparent background is achieved, only protein bands remain blue.
- For higher sensitivity, Silver staining can be applied which detect proteins in nanogram range; but this method is more complex and sensitive to contamination.
- In Silver method, fixation done first in ethanol/acetic acid, then gel treated with silver nitrate solution and developed with formaldehyde solution.
- Some researchers also used fluorescent dyes or radioactive labeling for more specific detection under UV or autoradiography, respectively.
- After completion of detection step, the gel is washed with distilled water and then preserved by drying or storing in plastic sheet for record purpose.
- The clarity of bands, contrast and sharpness depend on staining efficiency and gel handling during the process etc.
SDS PAGE vs native PAGE
- Nature of proteins– In SDS–PAGE, proteins are denatured by sodium dodecyl sulfate and reducing agents so that only primary structure remains, while in Native PAGE the proteins stay in their natural folded form with biological activity preserved.
- Charge factor– In SDS– PAGE, all proteins are coated with SDS which gives uniform negative charge, so migration depend mainly by molecular weight. In Native PAGE, charge depend on inherent net charge of protein and also its size and shape.
- Separation basis– SDS–PAGE separates proteins by size only, but Native PAGE separate by both charge/mass and conformation difference.
- Use of detergents – SDS– PAGE uses detergents like SDS, β-mercaptoethanol or DTT for denaturation, whereas Native PAGE no detergent or reducing agent used at all.
- Mobility pattern– In SDS type, smaller proteins move faster through gel pores, but in native type, migration vary irregularly depending on charge and structure of molecule.
- Molecular weight estimation – SDS–PAGE allows accurate molecular weight determination by comparing with standard marker, however in native it cannot estimate molecular weight precisely.
- Protein activity –Enzymatic or functional properties of proteins are lost in SDS–PAGE due to denaturation, while they are retained in native PAGE.
- Gel composition– Both methods use polyacrylamide gels, but buffer systems and sample preparations differ significantly.
- Visualization– Staining methods like Coomassie or silver stain used in both, but in native PAGE activity staining can also performed since proteins still active.
- Applications– SDS – PAGE mainly used for molecular weight analysis, purity check and protein profiling. Native PAGE used for studying enzyme activity, protein–protein interaction, and multimeric state etc.
- Appearance of bands– Bands in SDS–PAGE are sharp and well defined since charge uniform, while in native PAGE bands appear diffused due to difference in charge density.
- Complex proteins– In SDS –PAGE, multimeric proteins are separated into subunits, but in native PAGE they migrate as complete complexes.
- Summary– SDS–PAGE gives quantitative molecular data, native PAGE gives qualitative structural and functional information, both methods complement each other in protein study.
What is 2D gel electrophoresis?
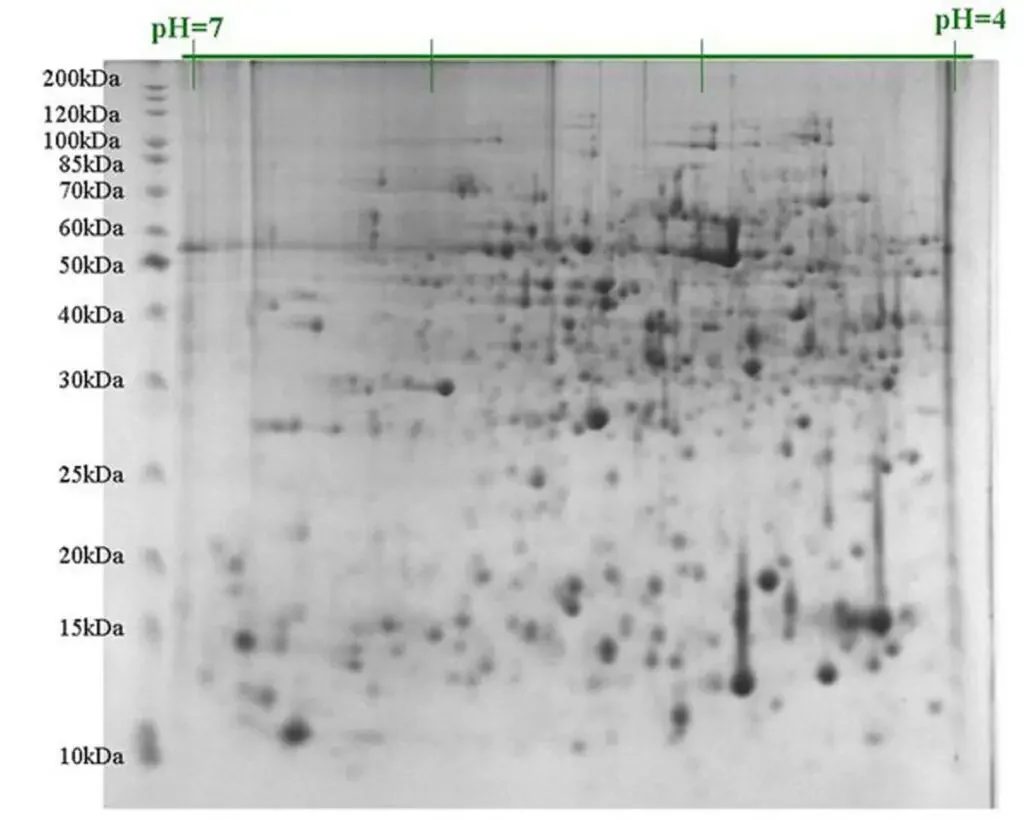
- 2D gel electrophoresis (2D-GE) refers as a advanced technique used for separation of proteins depend on two distinct properties – isoelectric point (pI) and molecular weight (Mw).
- In first dimension, proteins are separated by isoelectric focusing (IEF) where they migrate until reach at their pI value.
- The second dimension is carried out by SDS–PAGE, in which proteins separated according to their size/molecular weight.
- This method allow high resolution of complex protein mixtures, from cells or tissues, etc.
- The gel from first step (IEF strip) is placed horizontally on top of SDS–PAGE gel to perform second run, which make the 2D separation.
- Proteins are visualized by staining methods like Coomassie Brilliant Blue, silver stain, or fluorescent dyes.
- In comparison to single dimensional PAGE, 2D-GE give better distinguish between proteins having same Mw but different pI, or vice versa.
- It is used widely in proteomics studies for protein profiling, post-translational modification analysis and to compare expression between samples.
- The accuracy depend by various factors – buffer composition, pH gradient stability, gel uniformity, and proper sample preparation.
- Sometimes streaking or protein loss prevail the quality of result, especially for hydrophobic or very acidic/basic proteins.
- Data obtained are often analyzed by computer softwares which match and quantify protein spots automatically, but manual inspection also required often.
- Though technique is time-consuming and require expertise, it remain a sturdy and hardy method for proteome analysis.
Applications of Polyacrylamide Gel Electrophoresis (PAGE)
- Measurement of molecular weight of proteins is often done by PAGE (especially SDS‑PAGE) since proteins are separated by size when denatured.
- Estimation of protein purity is allowed by PAGE, because a single band suggests high purity and multiple bands indicate mixture.
- Analysis of protein subunits or aggregation state is facilitated by native-PAGE (non-denaturing) where the proteins keep some of their structure or complexes and their mobility vary by both size & charge.
- Comparison of polypeptide composition between samples is enabled by running two or more samples side by side in PAGE, then visualizing differences in band patterns.
- Preparation for downstream techniques: After PAGE separation, proteins are often transferred to membranes for Western blotting, or excised for mass-spec analysis or peptide mapping.
- For nucleic acids: In specific forms of PAGE (for example, acid-urea PAGE) the different forms of tRNA, or small RNAs with modifications, are separated by charge/shape differences.
- For forensic / biotechnology uses: The PAGE matrix allows high resolution separation of macromolecules (proteins / small DNA fragments) and hence it is used in forensic labs, biotech-labs for DNA- or protein-based analysis.
- Monitoring of protein integrity (for example checking for degradation, fragmentation) is allowed, by observing appearance/disappearance of expected bands or presence of unexpected bands.
- Estimation of protein quantitation by densitometry of bands after staining (though approximate) is another application.
- Separation of small nucleic acid fragments (for example oligonucleotides, very short DNA or RNA) is allowed with PAGE because the small pore size gives better resolution than agarose gel.
Advantages of Polyacrylamide Gel Electrophoresis (PAGE)
- A chemically cross-linked gel matrix is provided by PAGE, which gives stable and sturdy and hardy separation medium.
- Very high resolving power is offered, so molecules differing slightly in size or charge can be separated.
- The pore size of the gel can be easily adjusted (by changing acrylamide / bis-acrylamide concentrations) so that small fragments or proteins are resolved better.
- Recovery of sample from gel (for further use) is achieved with good purity when PAGE is used for DNA or proteins.
- Suitable for separation of small nucleic acid fragments (for DNAs of few base-pairs) or low molecular weight proteins, which other gels (like agarose) cannot resolve well.
- The matrix is chemically inert / electrically neutral / hydrophilic which makes detection (like staining) and optical visualization easy.
- It works for both proteins and nucleic acids (in many variants) so versatility is given.
Limitations of Polyacrylamide Gel Electrophoresis (PAGE)
- Preparation of the gel is more time‐consuming and complicated compared with simpler matrices, and the casting or handling of the gel are often tedious and error-prone.
- Toxicity of un-polymerized monomer (acrylamide) is a concern so safety measures must be used, which adds extra work.
- The technique is less suited for very large or very small molecules in some cases because the pore size and sieving effect become limiting, so only a certain range of sizes are resolved well.
- Quantitative accuracy is limited because staining, loading, gel variability etc. influence the band intensity and migration hence precise quantitation is hard.
- Throughput is fairly low: a gel run, staining, destaining, analysis take many steps and long time, so it’s not ideal for very high volume screening.
- Recovery of molecules (especially very hydrophobic or extreme pI proteins) is more difficult and some species may fail to enter the gel or get lost, so sample bias might be introduced.
FAQ
What is Polyacrylamide Gel Electrophoresis (PAGE)?
Polyacrylamide Gel Electrophoresis (PAGE) is a technique used to separate and analyze proteins or nucleic acids based on their size, charge, and shape using a polyacrylamide gel matrix.
What is the purpose of PAGE?
The main purpose of PAGE is to separate biomolecules, such as proteins or nucleic acids, into individual components based on their mobility in an electric field, allowing for further analysis and characterization.
How does PAGE work?
In PAGE, an electric field is applied to the gel matrix containing the biomolecules. The charged biomolecules migrate through the gel matrix at different rates based on their size and charge. Smaller molecules move faster, while larger ones move more slowly.
What are the different types of PAGE?
There are two main types of PAGE: native PAGE and denaturing PAGE. Native PAGE preserves the natural conformation of biomolecules, while denaturing PAGE disrupts the structure, typically using detergents or denaturing agents.
What is the role of acrylamide in PAGE?
Acrylamide is a key component in the gel matrix of PAGE. It polymerizes to form a stable gel matrix that provides a sieving effect, allowing for the separation of biomolecules based on size.
What is the difference between resolving gel and stacking gel in PAGE?
In PAGE, the gel matrix is divided into two regions: the resolving gel and the stacking gel. The resolving gel has a higher concentration of acrylamide and is responsible for separating biomolecules based on their size. The stacking gel has a lower concentration of acrylamide and helps to concentrate and focus the biomolecules before they enter the resolving gel.
How is protein size determined in PAGE?
Protein size is determined in PAGE by comparing the migration distance of protein bands on the gel to the migration of known molecular weight markers. A calibration curve can be constructed to estimate the size of unknown proteins.
Can PAGE be used for nucleic acid separation?
Yes, PAGE can be used for nucleic acid separation as well. It is commonly used for analyzing DNA fragments, RNA molecules, and other nucleic acid species.
What are the limitations of PAGE?
PAGE can be time-consuming and requires careful preparation. It may also have limitations in resolving very large or small biomolecules, and specialized techniques may be needed for complex samples.
What are the applications of PAGE?
PAGE has various applications in molecular biology, biochemistry, and biotechnology. It is used for protein purification, protein characterization, DNA sequencing, analysis of gene expression, and the study of protein-protein interactions, among others.
- https://vlab.amrita.edu/?sub=3&brch=186&sim=319&cnt=2
- https://conductscience.com/polyacrylamide-gel-electrophoresis/
- https://www.technologynetworks.com/analysis/articles/polyacrylamide-gel-electrophoresis-how-it-works-technique-variants-and-its-applications-359100
- https://www.news-medical.net/life-sciences/What-is-Polyacrylamide-Gel-Electrophoresis-(PAGE).aspx
- https://www.kharagpurcollege.ac.in/studyMaterial/115412Polyacrylamide%20and%20its%20electrophoresis,%20by%20Dr.Indranil%20Chakraborty.pdf
- https://www.bio-rad.com/webroot/web/pdf/lsr/literature/Bulletin_6040.pdf
- https://clinicalproteomicsjournal.biomedcentral.com/articles/10.1186/1559-0275-11-16
- https://openwetware.org/wiki/Polyacrylamide_gel_electrophoresis
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.