What is Zwitterion?
- A zwitterion, also known as an inner salt or dipolar ion, is a molecule in chemistry that contains an equal number of positively and negatively charged functional groups. The term “zwitterion” originates from the German word “zwitter,” which roughly translates to “hermaphrodite” or “hybrid.” It refers to the dual nature of these ions, possessing both positive and negative charges.
- Zwitterions are primarily electrically neutral, meaning their net formal charge is typically zero. This neutrality arises due to the balanced presence of positive and negative charges within the molecule. In aqueous solutions, zwitterions can exist in an equilibrium state with their parent molecules, where they interchange between the two forms.
- A notable example of zwitterions can be found in amino acids. When amino acids are dissolved in a solution, they can undergo an equilibrium reaction that produces zwitterions. These zwitterions play a crucial role in biological systems, such as protein structures and enzyme catalysis.
- While zwitterions are generally electrically neutral, there are exceptions. Betaines are a type of zwitterion that cannot isomerize to an all-neutral form, particularly when the positive charge is located on a quaternary ammonium group. Similarly, a molecule containing a phosphonium group and a carboxylate group cannot undergo isomerization.
- In medicinal chemistry, zwitterions can be significant in the design of pharmaceutical compounds. When working with acid, basic, or neutral leads, zwitterions may offer unique properties or interactions that can be exploited for therapeutic purposes. Understanding the behavior and characteristics of zwitterions allows researchers to make informed decisions during the drug discovery and development process.
- In summary, a zwitterion is a molecule that contains both positive and negative charged functional groups. It is often electrically neutral and plays a vital role in various chemical and biological processes. The term “zwitterion” reflects its dual nature, derived from the German word “zwitter,” meaning “hermaphrodite” or “hybrid.” Zwitterions have implications in fields such as biochemistry and medicinal chemistry, where their properties and behavior are studied and utilized for different applications.
Zwitterion Definition
A zwitterion is a molecule that contains both positively and negatively charged functional groups, resulting in an overall neutral charge.
Properties of Zwitterion
Zwitterions exhibit several distinct properties due to their unique structure and combination of positively and negatively charged ions. Here are some key properties of zwitterions:
- Dual Charged Structure: Zwitterions contain both positively charged (cationic) and negatively charged (anionic) functional groups within the same molecule. This dual charged structure arises from the presence of acid or base groupings in the compound. Ampholytes are a common example of zwitterionic compounds that possess both acidic and basic functional groups.
- Covalent Bonding: The positively and negatively charged ions in zwitterions are held together through covalent bonding. This covalent bond ensures that the charged groups remain attached to the molecule and do not dissociate easily.
- Net Neutral Charge: Despite containing both positive and negative charges, zwitterions are electrically neutral overall. The presence of equal and opposite charges within the molecule cancels out the net formal charge, resulting in electrical neutrality. This property contributes to the stability of zwitterions.
- Charge Separation: Zwitterions maintain the separation of positive and negative charges within the molecule. The covalent bonds between the charged groups and the rest of the molecule ensure that the unit electrical charges are kept apart from the atoms. This charge separation plays a crucial role in the stability and unique properties of zwitterions.
- Ammonium Cations: Many zwitterions, particularly those derived from amino acids, feature ammonium cations. Ammonium cations are positively charged ions consisting of a central nitrogen atom bonded to four hydrogen atoms. These cations contribute to the positive charge within zwitterions and play a significant role in their overall chemical behavior.
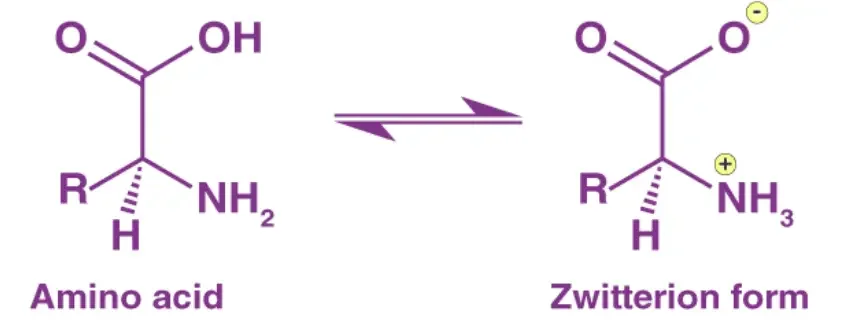
Zwitterion Structure
- The zwitterion structure is primarily observed in amino acids, although it can also occur in other compounds that possess acid and base centers. The most common example of a zwitterion is found in amino acids, where they exhibit a dual charged structure.
- Amino acids consist of an ammonium or amino group (-NH3+) that carries a positive charge and a carboxyl group (-COO-) that carries a negative charge. These two functional groups are attached to a central carbon atom, which is also bonded to a hydrogen atom and a side chain specific to each amino acid. The zwitterion form of an amino acid is a result of the internal ionization of these functional groups, leading to the presence of both positive and negative charges within the same molecule.
- Apart from amino acids, other compounds can also adopt the zwitterion form if they contain acid and base centers. Examples of such compounds include tricine, bicine, solid sulfamic acid, and alkaloids like psilocybin, among others. These compounds exhibit the characteristic zwitterion structure due to the presence of both positive and negative charges within their chemical composition.
- In summary, the zwitterion structure is commonly observed in amino acids, where the presence of an ammonium group and a carboxyl group results in a molecule with both positive and negative charges. However, other compounds with acid and base centers can also exhibit the zwitterion form. Understanding the zwitterion structure is important in various fields of chemistry and biochemistry, as it influences the behavior and properties of these compounds.
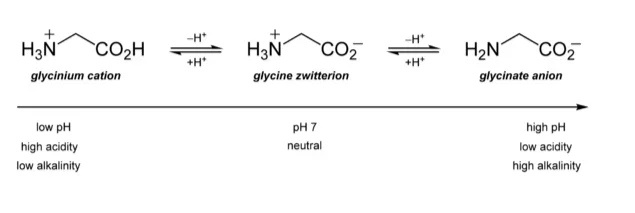
Isoelectric Point
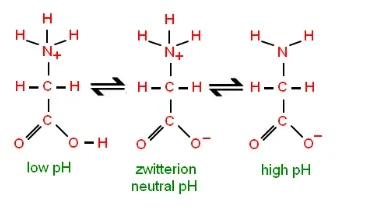
- The isoelectric point (pI) is a significant property associated with zwitterions. It represents the pH value at which the overall charge of the molecule is neutral. The pI is determined by the equilibrium between the positive and negative charges within the zwitterion.
- The net charge on a zwitterionic molecule is greatly influenced by the pH of its surrounding environment. At lower pH values, the excess of protons in the solution can interact with the zwitterion, resulting in a net positive charge as the amino group acts as a proton acceptor. Conversely, at higher pH values, the excess of hydroxide ions can interact with the zwitterion, resulting in a net negative charge as the carboxyl group acts as a proton donor.
- The isoelectric point is the specific pH at which the positive and negative charges within the zwitterion are balanced, resulting in a neutral overall charge. At this pH, the zwitterion exists in its most stable form. Above or below the pI, the zwitterion will carry a net positive or net negative charge, respectively.
- Furthermore, the pI value also influences the solubility of the molecule in a given pH environment. At a pH below the pI, zwitterionic molecules tend to be more soluble due to the prevalence of positive charges that can interact with the surrounding solvent. Conversely, at a pH above the pI, the zwitterionic molecules tend to be less soluble as the prevalence of negative charges reduces the interactions with the solvent.
- The knowledge of the pI value of a zwitterionic molecule is crucial in various fields, including biochemistry and pharmaceutical sciences. It helps in understanding the behavior and properties of the molecule in different pH environments, and it can assist in optimizing the solubility, stability, and interactions of zwitterionic compounds for various applications.
- In summary, the isoelectric point (pI) is the pH value at which a zwitterion carries a neutral charge. The pI is influenced by the equilibrium between positive and negative charges within the zwitterion, and it affects the solubility of the molecule at different pH levels. Understanding the pI value is essential for studying the behavior and properties of zwitterionic compounds in various scientific disciplines.
Calculation of pH Value
The pH value at the isoelectric point of a zwitterion can be calculated using the equilibrium constants of its acidic and basic groups. The formula to determine the pI is as follows:
pI = (pKa1 + pKa2) / 2
In this equation:
- pI represents the isoelectric point, which is the pH value at which the zwitterion carries a neutral charge.
- pKa1 refers to the equilibrium constant of the acidic group present in the zwitterion.
- pKa2 represents the equilibrium constant of the basic group within the zwitterion.
The pKa values are logarithmic representations of the equilibrium constants, which describe the strength of the acid or base. They provide information about the tendency of the acidic or basic group to donate or accept protons, respectively.
To calculate the pI, the pKa values of the acidic and basic groups are added together and then divided by 2. This calculation yields the pH value at which the positive and negative charges within the zwitterion are in equilibrium, resulting in a neutral overall charge.
It is important to note that the pKa values can vary depending on the specific zwitterionic compound and the environmental conditions. Therefore, the calculated pI is an approximation and may not precisely represent the actual isoelectric point in some cases.
By calculating the pH value at the isoelectric point, scientists can gain insights into the behavior, charge distribution, and stability of zwitterionic molecules in different pH environments. This information is valuable in various fields, including biochemistry, pharmaceutical sciences, and protein chemistry.
In summary, the pH value at the isoelectric point of a zwitterion can be determined using the pKa values of its acidic and basic groups. The pI is calculated by taking the average of the pKa values using the formula (pKa1 + pKa2) / 2. This calculation provides an estimation of the pH value at which the zwitterion carries a neutral charge.
Applications of Zwitterions
Zwitterions have found numerous applications in various fields due to their unique properties and versatility. Here are some notable applications of zwitterions:
- Protein Separation: Zwitterions play a crucial role in the SDS PAGE method, which is widely used in molecular biology to separate protein molecules based on their size. The presence of zwitterionic detergents, such as sodium dodecyl sulfate (SDS), helps to denature and coat proteins uniformly, enabling their separation during electrophoresis.
- Medical and Biological Applications: Zwitterions have significant potential in medical and biological fields. They are utilized in medical implants, where zwitterionic coatings help reduce inflammation and enhance the compatibility of implants with the body’s tissues. Zwitterionic materials are also explored for drug delivery systems, as they can improve the stability and targeted release of drugs. Additionally, zwitterionic coatings on blood contact sensors and separation membranes contribute to their biocompatibility and reduced fouling.
- Antifouling Coatings: Zwitterionic polymers have been employed as antifouling coatings in biomedical implants. These coatings prevent the adhesion of microbial organisms and the formation of biofilms, which can lead to infections and complications. Zwitterionic coatings provide a repulsive force against microbes, reducing the risk of biofouling and improving the longevity and performance of implants.
- Marine Industry: Zwitterionic polymers are utilized in the marine industry to prevent the accumulation of subaquatic organisms on boats, piers, and other marine structures. By incorporating zwitterionic coatings, these materials resist the attachment and growth of marine organisms, thereby reducing drag, enhancing fuel efficiency, and extending the lifespan of marine structures.
These applications highlight the diverse uses of zwitterions in various scientific and industrial domains. The unique properties of zwitterions, such as their biocompatibility, resistance to fouling, and surface repulsion, make them valuable in fields ranging from biomedicine to marine engineering. Continued research and development in zwitterionic materials are expected to lead to further advancements and novel applications in the future.
Examples of Zwitterions
There are several examples of zwitterions found in various chemical compounds. Here are some notable examples:
- Amino Acids: Amino acids are perhaps the most well-known examples of zwitterions. They contain both an amino group (-NH3+) and a carboxyl group (-COO-) within the same molecule, resulting in a zwitterionic structure. Examples of amino acids include glycine, alanine, lysine, and glutamic acid.
- Betaines: Betaines are a class of zwitterionic compounds that cannot isomerize to an all-neutral form. They possess a positively charged quaternary ammonium group and a negatively charged carboxylate group. Examples of betaines include betaine itself (trimethylglycine), cocamidopropyl betaine (used in personal care products), and glycine betaine (found in certain plants and animals).
- Phospholipids: Phospholipids are essential components of biological membranes. They consist of a hydrophilic head group, which is often zwitterionic, and hydrophobic fatty acid tails. Examples of zwitterionic phospholipids include phosphatidylcholine and phosphatidylserine.
- Sulfobetaines: Sulfobetaines are zwitterionic compounds that contain both a positively charged quaternary ammonium group and a negatively charged sulfonate group. They are known for their excellent water solubility and biocompatibility. Examples of sulfobetaines include N,N-dimethyl-N-(3-sulfopropyl) ammonium betaine (SPAB) and N,N-dimethyl-N-(3-sulfopropyl)-2-(trimethylammonio) ethanaminium (SB3-14).
- Taurine: Taurine is an organic compound that serves as a zwitterion. It contains an amino group (-NH2) and a sulfonic acid group (-SO3H). Taurine is found in high concentrations in mammalian tissues, particularly in the heart, muscles, and central nervous system.
These are just a few examples of zwitterions commonly encountered in chemistry and biology. Zwitterionic compounds can be found in various natural and synthetic substances, and their unique properties and structures make them valuable in numerous applications.
FAQ
What is a zwitterion?
A zwitterion is a molecule or ion that contains both positively and negatively charged groups, resulting in overall electrical neutrality.
How does a zwitterion form?
Zwitterions form when a molecule or ion has functional groups with both acidic and basic properties. The presence of these groups allows for the simultaneous donation and acceptance of protons, resulting in the formation of positive and negative charges.
What are some examples of zwitterions?
Examples of zwitterions include amino acids, betaines, phospholipids, sulfobetaines, and taurine.
What is the significance of the isoelectric point (pI) of a zwitterion?
The isoelectric point is the pH at which a zwitterion carries no net charge. It is an important property that influences the behavior, solubility, and stability of zwitterionic compounds.
How is the pI of a zwitterion calculated?
The pI is calculated by averaging the pKa values of the acidic and basic groups within the zwitterion. The formula is typically expressed as (pKa1 + pKa2) / 2.
What are the applications of zwitterions?
Zwitterions have various applications, including protein separation, medical implants, drug delivery systems, blood contact sensors, separation membranes, and antifouling coatings in biomedical and marine industries.
How do zwitterions prevent fouling in biomedical implants?
Zwitterionic coatings on implants create a repulsive force against microbial organisms, reducing the adhesion and formation of biofilms, thus preventing fouling and improving the longevity of implants.
Are zwitterions biocompatible?
Yes, zwitterions are known for their biocompatibility. Their electrically neutral nature and low tendency for protein adsorption make them suitable for biomedical applications.
Can zwitterions be used in drug delivery systems?
Yes, zwitterions have shown promise in drug delivery systems. Their stability, solubility, and ability to interact with specific target sites can enhance the efficiency and controlled release of drugs.
Are all amino acids zwitterions?
Yes, all naturally occurring amino acids are zwitterions due to the presence of both an amino group (positive charge) and a carboxyl group (negative charge) in their chemical structures.