Loeffler’s methylene blue staining is a simple staining method used mainly for observing the morphology of Corynebacterium diphtheriae. It is the process where Loeffler’s alkaline methylene blue solution is applied on a fixed smear, and this stain contains a small amount of potassium hydroxide (KOH) which makes the solution alkaline.
It is the alkalinity that increases the negative charge on different cell components like nucleic acids and proteins, so the basic dye binds more strongly. The method is important because it shows metachromasia, and this is referred to as the appearance of deep blue-black or bluish-purple granules called volutin or Babes-Ernst granules inside the pale blue cytoplasm of the organism.
These granules is the polymerised polyphosphate stored inside the cells. The stained cells often appear as beaded rods arranged at angles, sometimes looking like L, V or in Chinese-letter pattern. In this method, a thin smear is prepared and fixed, then it is flooded with the stain for about 1–3 minutes, washed with water and examined under oil immersion. It is considered a rapid technique because the stained smear can be observed almost immediately. It is also used as the blue counterstain in acid-fast staining procedures where the non–acid-fast cells take the blue colour.
What do you means by Viability Staining?
Viability staining is the process in which special dyes are used to differentiate the living cells from the dead cells in a sample. It is a rapid method and does not depend on culture, so it helps to detect those microorganisms that remain viable but cannot be cultured easily. In this method the differentiation is mainly based on membrane integrity or metabolic activity.
The dyes that enter all cells stain them uniformly, while the dyes that enter only damaged cells indicate the non-viable population. It is the process where stains like SYTO 9, Propidium Iodide (PI) or Trypan Blue are commonly used, and the cells with intact membrane appear differently from the cells with damaged membrane. Some of the viability stains detect the metabolic activity of the cell by reduction of tetrazolium salts or by hydrolysis of fluorescein diacetate (FDA), and these reactions occur only in active cells. The stained preparations are observed under fluorescence microscope, flow cytometer or other instruments for quick analysis of viable and non-viable cells in the sample.
Aim
To distinguish between living and dead cells.
Principle of Viability Staining by Loeffler’s Method
The principle of viability staining by Loeffler’s method is based on the way methylene blue reacts differently with living and dead cells. It is the process in which Loeffler’s alkaline methylene blue solution enters the cell without the need of heat fixation, because the stain acts as a supravital dye that can penetrate cells which are still alive.
The dye is strongly cationic, so it binds with the negatively charged components like nucleic acids and polyphosphates, and the alkaline nature of the stain increases this negative charge, making the binding more effective. In living cells, there is active metabolism, and these cells can reduce methylene blue into a colourless form.
Because of this enzymatic reduction, the viable cells remain either colourless or show a very faint purple appearance. The dead cells cannot reduce the dye, so methylene blue stays in its coloured form inside them, making the dead cells appear blue. It is this difference in metabolic ability that is used to distinguish the viable cells from the non-viable ones in the stained preparation.
Requirement for Viability Staining by Loeffler’s Method
- Loeffler’s methylene blue solution (alkaline).
- Dilute carbol fuchsin solution used as the counterstain.
- Bacterial culture about 2–3 weeks old for preparing the smear.
- Clean glass slide for preparing the unfixed smear.
- Bunsen burner used only for sterilising the loop, not for fixing the smear.
- Inoculating loop for transferring the culture.
- Blotting paper for drying the stained preparation.
- Microscope for observing the viable and non-viable cells.
- Buffer like PBS in which the cells can be suspended before staining.
- Stock methylene blue prepared by dissolving the dye in sodium citrate solution (0.1 mg/mL in 2% sodium citrate).
- Incubation of the smear with methylene blue for a short time (about 5 minutes) at room temperature.
- Alkaline condition maintained by diluted KOH that increases the negative charge on the cell components.
- Unfixed smear required so that the dye can act as a supravital stain and penetrate the living cells directly.
Procedure of Viability Staining by Loeffler’s Method
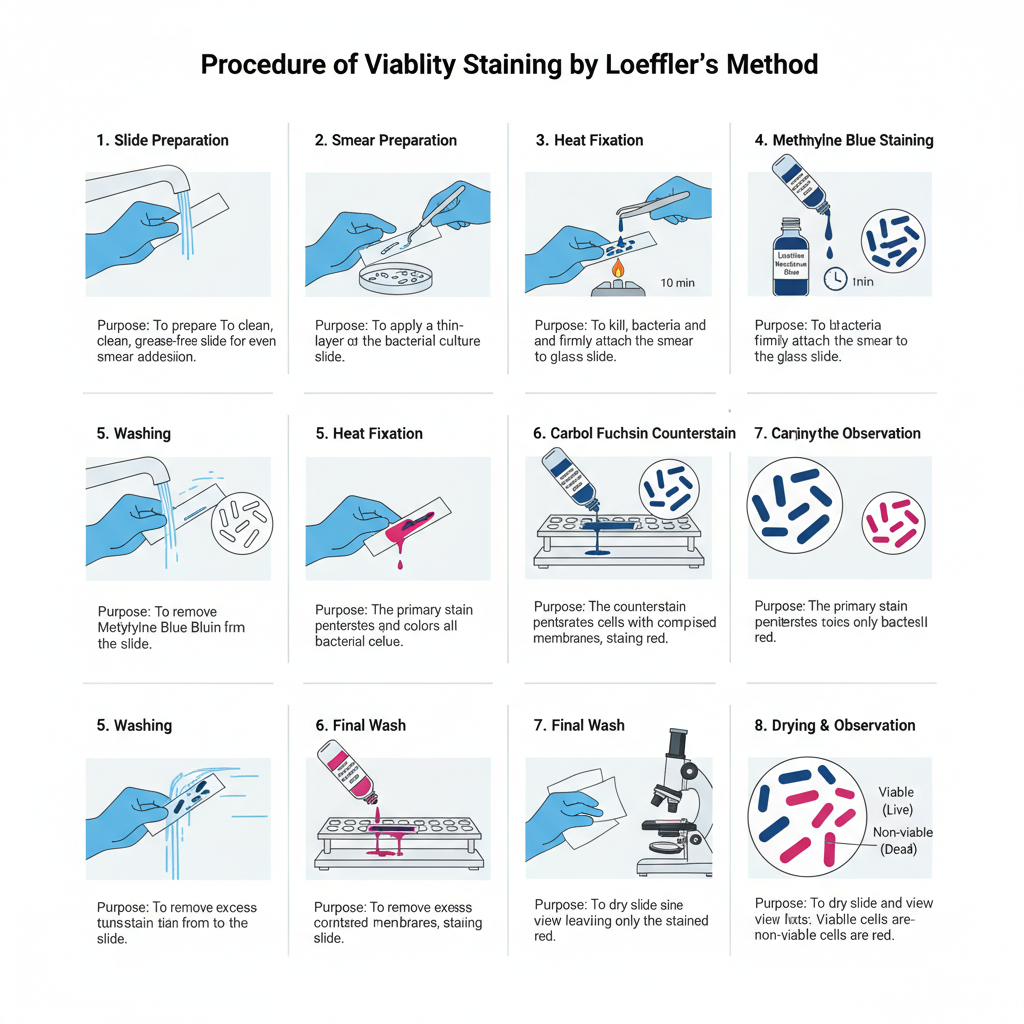
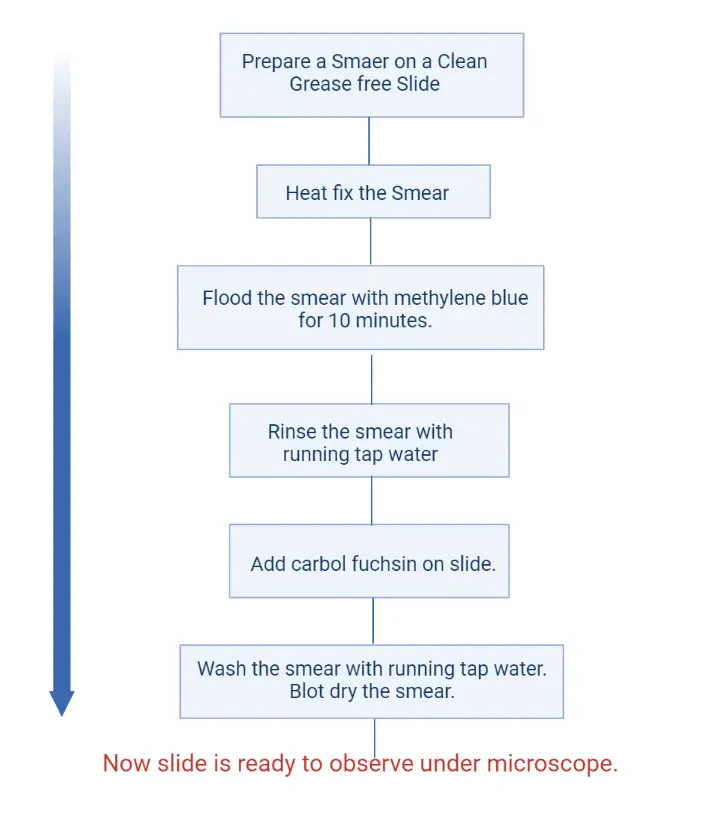
- Preparation of Slide– A clean glass slide is taken and washed properly with soap and water. It is then allowed to dry. The slide must be grease-free for proper staining.
- Smear Preparation– The inoculating loop is sterilised by heating until it becomes red hot and then cooled. The cooled loop is used to transfer the bacterial culture (generally 2–3 week old culture) to the slide. The material is spread to form a thin smear.
- Heat Fixation– The slide is passed over the flame 2–3 times. It is the process that fixes the bacterial smear on the slide surface.
- Methylene Blue Staining– The fixed smear is covered with Loeffler’s methylene blue solution. It is kept for 10 minutes so that the dye can enter the cells.
- Washing– The slide is washed with running water until a pale blue colour is seen on the smear.
- Carbol Fuchsin Counterstain– The smear is flooded with carbol fuchsin. This stain is added directly after washing.
- Washing– The slide is washed immediately with tap water to remove the extra stain.
- Drying and Observation– The slide is dried with blotting paper. It is then ready for viewing under the microscope.
Viability Staining Method For Bacteria Flow Chart
Result of Viability Staining
Live Bacterial Cells – These appear purple. It is the process where living cells reduce methylene blue into a colourless compound, and then the carbol fuchsin counterstain give the purple appearance.
Dead Bacterial Cells – These appear red or pink because dead cells do not reduce the dye. In some descriptions dead cells may also appear blue when methylene blue is fully retained.
Living Spores – Stained pink.
Dead Spores – Stained blue.
Uses of Loeffler’s Method
- It is used to differentiate living cells and dead cells in a bacterial population based on the colour reaction after staining.
- It is the process that helps to assess the metabolic activity of the cells because only the viable cells reduce the methylene blue dye.
- It is applied for rapid viability testing when results are needed within minutes without using culture methods.
- It allow microscopic observation of individual cells so that the percentage of live and dead cells can be estimated directly.
- It gives a more objective idea about the cell death rate because it can identify viable but non-culturable cells which may not grow on culture media.
- It is used for checking the effectiveness of antimicrobial agents or disinfectants by observing the live and dead organisms after treatment.
- It can be used for determining microbial activity in different samples like environmental specimens, food materials, and laboratory cultures.
Advantages of Loeffler’s Method
- It is a rapid method and the results are obtained within minutes, unlike culture-based methods that take long time for observing viability.
- It allow direct visualization of individual cells under the microscope, so living and dead cells can be easily distinguished.
- The method is simple and low-cost because methylene blue is a basic dye and no advanced instruments are required for the staining.
- It is the process that detects metabolically active cells as the living cells reduce the dye due to the presence of intracellular enzymes.
- It can show the viable but non-culturable cells which may not produce colonies in culture methods but still reduce the dye.
- It is useful when quick estimation of viability percentage is required in bacterial population.
Limitations of Loeffler’s Method
- It measures vitality of the cells, not the true viability, because the method depend on the metabolic activity for reducing the dye.
- It may overestimate the living population as cells that cannot reproduce may still reduce the dye and appear viable.
- It cannot detect dormant or non-metabolic cells because these cells do not show enzyme activity for dye reduction.
- It needs careful timing since long exposure to methylene blue may kill the cells and produce false-positive dead cell results.
- It becomes labor-intensive when many samples are handled because each slide must be viewed under the microscope.
- The staining results may vary depending on the number of bacteria and the amount of stain used in the smear.
- It is mainly suitable for pure cultures and show interference when used for environmental or food samples containing mixed materials.
- It cannot be used alone for accurate quantitative comparison and need validation with plating efficiency or other culture-based methods.
- Alsharif, R., Tapia, M., Godfrey, W., Wannlund, J., & Nagar, M. (n.d.). Bacterial disinfectant efficacy using flow cytometry. BD Biosciences.
- BD Biosciences. (n.d.). Cell viability kit.
- Biology Notes Online. (n.d.). Viability staining method for bacteria.
- Braissant, O., Astasov-Frauenhoffer, M., Waltimo, T., & Bonkat, G. (2020). A review of methods to determine viability, vitality, and metabolic rates in microbiology. Frontiers in Microbiology, 11, 547458. https://doi.org/10.3389/fmicb.2020.547458
- Berney, M., Hammes, F., Bosshard, F., Weilenmann, H.-U., & Egli, T. (2007). Assessment and interpretation of bacterial viability by using the LIVE/DEAD BacLight Kit in combination with flow cytometry. Applied and Environmental Microbiology, 73(10), 3283–3290. https://doi.org/10.1128/AEM.02750-06
- Cangelosi, G. A., & Meschke, J. S. (2014). Dead or alive: Molecular assessment of microbial viability. Applied and Environmental Microbiology, 80(19), 5884–5891. https://doi.org/10.1128/AEM.01763-14
- Cell Signaling Technology. (n.d.). Overview of cell viability and survival.
- Chiang, J., Robertson, J., McGoverin, C. M., Swift, S., & Vanholsbeeck, F. (2024). Rapid detection of viable microbes with 5-cyano-2,3-di-(p-tolyl)tetrazolium chloride and 5(6)-carboxyfluorescein diacetate using a fibre fluorescence spectroscopy system. Journal of Applied Microbiology, 135(3), lxae047. https://doi.org/10.1093/jambio/lxae047
- Davey, H. M. (2011). Life, death, and in-between: meanings and methods in microbiology. Applied and Environmental Microbiology, 77(17), 5571–5576.
- Frlolov, O. A., Terekhin, A. V., Yakushev, A. V., & Milanovskiy, E. Y. (2022). Method for determining microbial activity (hydrolysis of fluorescein diacetate (FDA), 490 nm). IOP Conference Series: Earth and Environmental Science, 1093(1), 012016. https://doi.org/10.1088/1755-1315/1093/1/012016
- Keer, J. T., & Birch, L. (2003). Molecular methods for the assessment of bacterial viability. Journal of Microbiological Methods, 53, 175–183. https://doi.org/10.1016/S0167-7012(03)00025-3
- Kim, J. S., & Nam, M. H. (2011). Comparison of the automated fluorescence microscopic viability test with the conventional and flow cytometry methods. Journal of Clinical Laboratory Analysis, 25(2), 90–94. https://doi.org/10.1002/jcla.20438
- Kwolek-Mirek, M., & Zadrag-Tecza, R. (2014). Comparison of methods used for assessing the viability and vitality of yeast cells. FEMS Yeast Research, 14(7), 1068–1079. https://doi.org/10.1111/1567-1364.12202
- Li, L., Mendis, N., Trigui, H., Oliver, J. D., & Faucher, S. P. (2014). The importance of the viable but non-culturable state in human bacterial pathogens. Frontiers in Microbiology, 5, 258. https://doi.org/10.3389/fmicb.2014.00258
- Netuschil, L., Auschill, T. M., Sculean, A., & Arweiler, N. B. (2014). Confusion over live/dead stainings for the detection of vital microorganisms in oral biofilms—which stain is suitable? BMC Oral Health, 14(2). https://doi.org/10.1186/1472-6831-14-2
- Ou, F., McGoverin, C., Swift, S., & Vanholsbeeck, F. (2019). Rapid and cost-effective evaluation of bacterial viability using fluorescence spectroscopy. Analytical and Bioanalytical Chemistry, 411(16), 3653–3663. https://doi.org/10.1007/s00216-019-01848-5
- Pinto, R. M., Soares, F. A., Reis, S., Nunes, C., & Van Dijck, P. (2020). Innovative strategies toward the disassembly of the EPS matrix in bacterial biofilms. Frontiers in Microbiology, 11, 952. https://doi.org/10.3389/fmicb.2020.00952
- ResearchGate. (n.d.). What is the limit of detection (CFU/mL) for bacterial viability stains? [Forum post].
- Samuel, B. J., Jin, Z., Merkel, G., Heider, V., Sharma, D. S., Proff, P. M., Epple, M. F., Müller, C. W., & Helling, B. K. (2025). Evaluating cell viability assessment techniques: a comparative study of flow cytometry and fluorescence microscopy in response to bioactive glass exposure. Journal of Biomedical Engineering, 24(112). https://doi.org/10.1186/s12938-025-01452-y
- Schottroff, F., Fröhling, A., Zunabovic-Pichler, M., Krottenthaler, A., Schlüter, O., & Jäger, H. (2018). Sublethal injury and viable but non-culturable (VBNC) state in microorganisms during preservation of food and biological materials by non-thermal processes. Frontiers in Microbiology, 9, 2691. https://doi.org/10.3389/fmicb.2018.02691
- Shehata, H. R., Pane, M., Buys, E. M., Koshy, B., Vegge, C. S., & Schoeni, J. L. (2025). Editorial: Emerging technologies for viability enumeration of live microorganisms. Frontiers in Microbiology, 15, 1546438. https://doi.org/10.3389/fmicb.2024.1546438
- Stiefel, P., Schmidt-Emrich, S., Maniura-Weber, K., & Ren, Q. (2015). Critical aspects of using bacterial cell viability assays with the fluorophores SYTO9 and propidium iodide. BMC Microbiology, 15(36). https://doi.org/10.1186/s12866-015-0376-x
- Tchatchiashvili, T., Jundzill, M., Marquet, M., Mirza, K., Pletz, M., Makarewicz, O., & Thieme, L. (2025). CAM/TMA-DPH as a promising alternative to SYTO9/PI for cell viability assessment in bacterial biofilms. Frontiers in Microbiology.
- Thermo Fisher Scientific. (n.d.). BacLight™ RedoxSensor™ Green Vitality Kit 200 kit | Buy Online | Invitrogen™.
- Wikipedia. (n.d.). Fluorescein diacetate hydrolysis.
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.