Vacuole staining is the process in which specific dyes are used to visualize the vacuole because this organelle is usually large and transparent, so it is not easily observed under normal light microscopy. It is the process where chemical dyes or fluorescent probes are taken up either by the tonoplast membrane or by the vacuolar lumen, and this helps in studying the morphology and physiological condition of the vacuole. Neutral Red is the most common dye used in living cells, and it is accumulated inside the acidic lumen.
This is referred to as an indicator of cell viability because the dye is trapped only when an active proton gradient is maintained. FM 4-64 is another dye used in staining, and it is the lipophilic styryl dye which first binds to the plasma membrane and then moves inward by endocytosis to reach the vacuolar membrane.
It is used to study membrane trafficking. Some fluorescent dyes like BCECF-AM stain the vacuolar lumen for pH measurement, while MDY-64 is used for yeast vacuole membrane. In metabolically active yeast cells, dyes such as FUN 1 are processed to form fluorescent structures inside the vacuole indicating cellular vitality. This process occurs when researchers need to observe events like contractile vacuole activity in protozoa or changes seen during osmotic stress in plant cells.
Principle Vacuole Staining
The principle of vacuole staining is based on the distinct physiological and chemical features of the vacuole, and it is the process where dyes accumulate either in the acidic lumen or on the tonoplast depending on their properties. It is the process referred to as acidotropic trapping when Neutral Red is used. In its unprotonated form the dye diffuses across membranes, but once it enters the acidic vacuolar lumen it becomes protonated and is retained because the charged form cannot pass out through the membrane. The accumulation is possible only when the cell maintains an active proton gradient, so staining indicates the viability of the cell and the integrity of the vacuole.
In another principle, styryl dyes such as FM 4-64 stain the vacuolar membrane by following the endocytic route. The dye first binds to the plasma membrane and then it is internalized into endosomes which later fuse with the vacuole. It is the process showing membrane trafficking in living cells. Some dyes like FUN 1 depend on the metabolic activity of the cell, and these dyes are converted into fluorescent structures inside the vacuole when the cell is active. Thus the staining is used to observe both the structural and the functional properties of the vacuole.
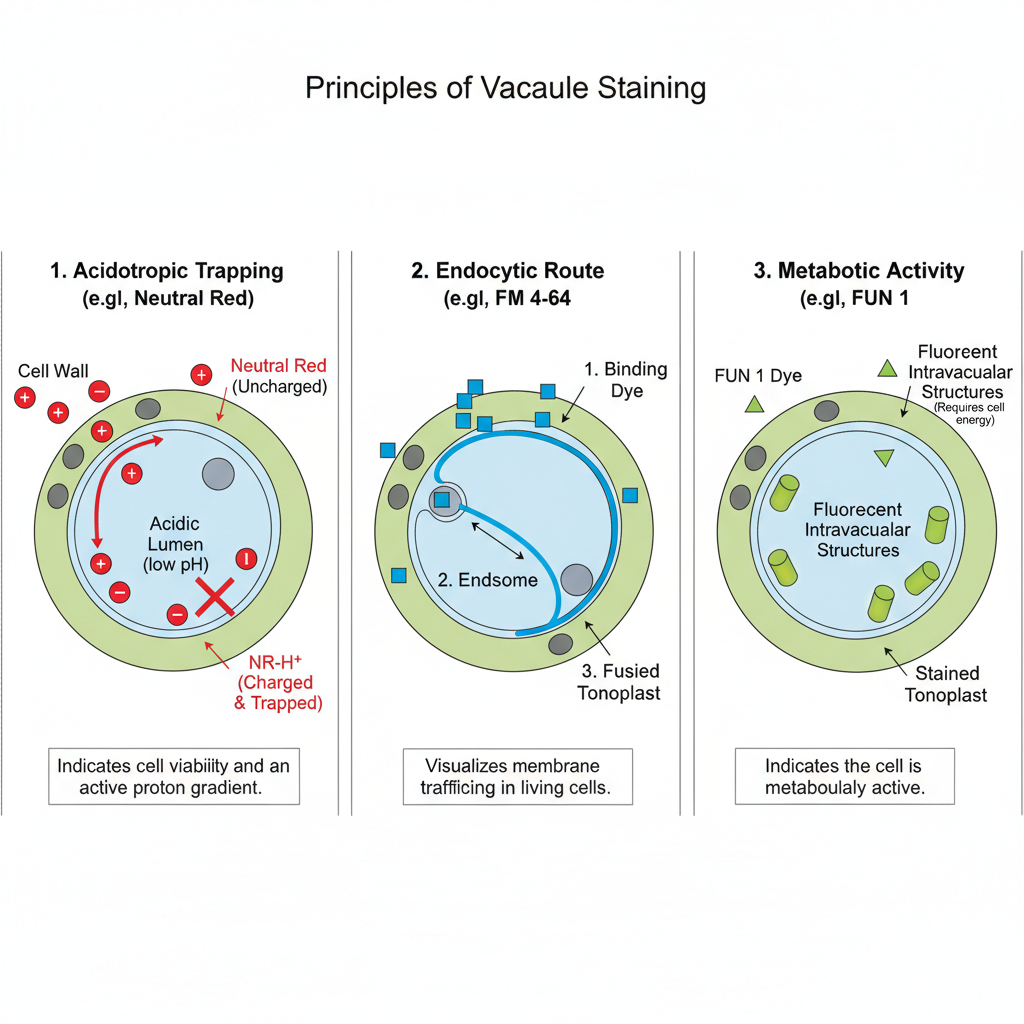
Requirements for Vacuole Staining
Some of the main requirements for Vacuole Staining are–
- Living cells must maintain an acidic vacuolar lumen for dyes like Neutral Red.
- Active proton pumps (V-ATPases) are needed because the dye is trapped only when a pH gradient is present.
- Cells should have intact membranes since dead cells show diffuse staining.
- Light exposure must be controlled during observation because Neutral Red can increase phototoxic effects.
- Physiological temperature is needed for FM 4-64 staining since endocytosis is required for the dye to reach the vacuole.
- Sufficient incubation time is needed for the dye to move from the plasma membrane into endosomes and finally the vacuole.
- Metabolically active cells are required when using FUN 1 because fluorescent structures are formed only when metabolism is active.
- In some plant tissues dyes like BCECF-AM require surfactants to help in dye uptake.
- Thin tissue layers are needed in plant samples so that the vacuole is clearly visible.
- Some dyes need a washout step to remove excess plasma membrane staining.
- Motile organisms need to be slowed or immobilized for clear observation of vacuoles.
- Fluorescent dyes generally require fluorescence or confocal microscopy for proper visualization of the tonoplast.
Procedure for Vacuole Staining
A. Classical Vital Staining: Neutral Red (Lumen & pH)
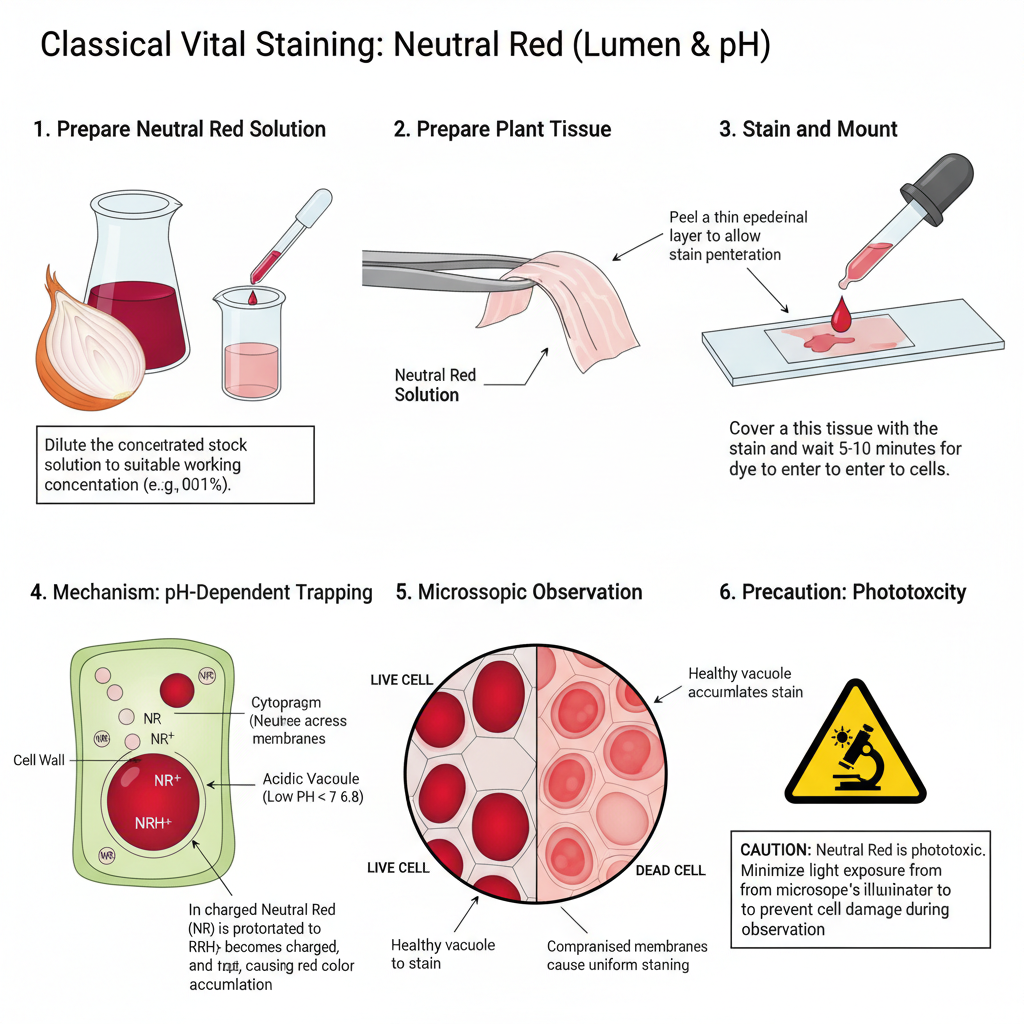
- Prepare the Neutral Red solution by making a stock and then dilute it to a suitable working concentration.
- Peel a thin single layer of plant epidermis so that the stain can enter and the vacuole is visible.
- Place the tissue sample on a slide for mounting.
- Add the diluted Neutral Red solution so that the tissue is fully covered.
- Keep the stain for some minutes because the dye must enter the cell and get trapped in the acidic vacuole.
- Remove the staining solution and rinse gently with water or buffer to clear excess dye from the wall and cytoplasm.
- Observe the sample under bright-field microscope where the vacuole appears red if the cell is alive.
- Live cells show deep red vacuoles, while dead cells show uniform staining of cytoplasm or nucleus.
- Light exposure should be kept low because Neutral Red can increase phototoxic effects.
B. Dynamic Membrane Tracking: FM 4-64 (Endocytosis)
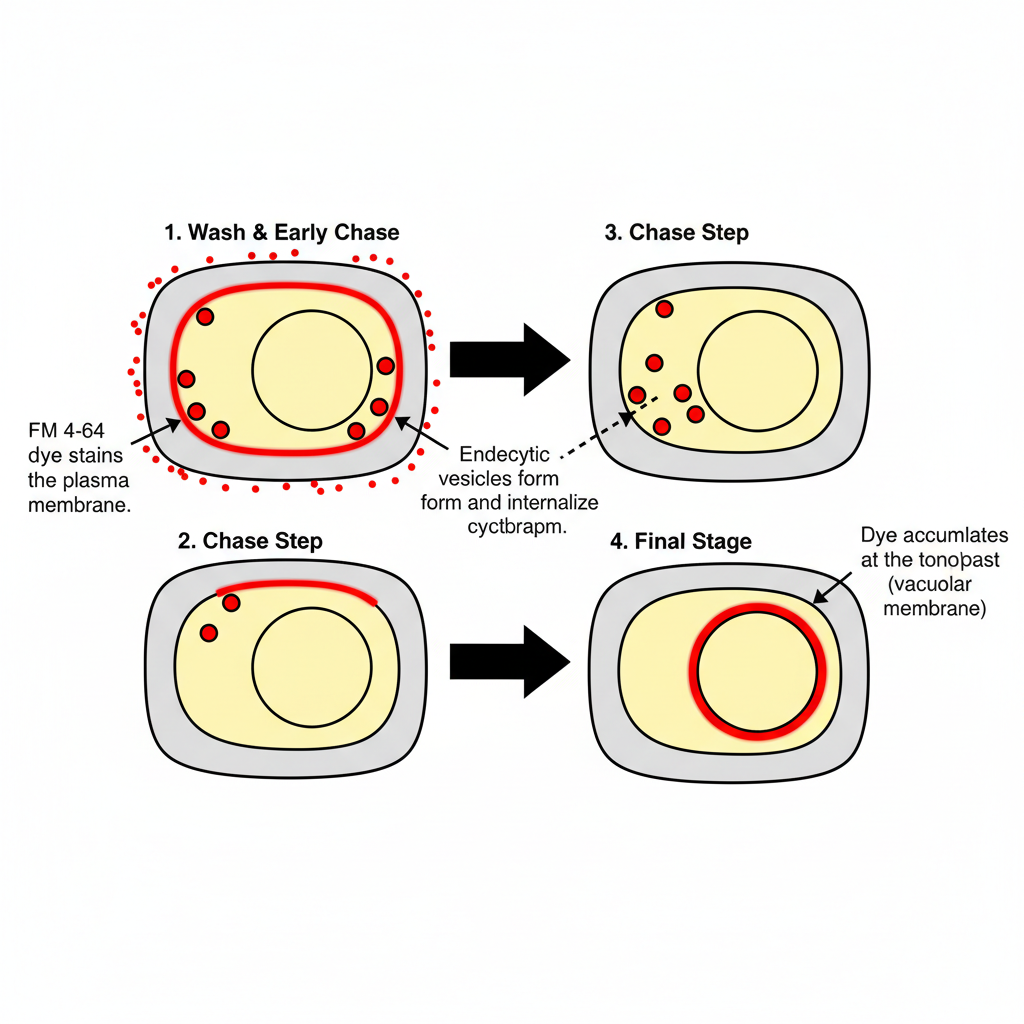
- Maintain the sample at physiological temperature because endocytosis is active only at this range.
- Prepare the FM 4-64 stock in DMSO and dilute it with suitable growth medium to make the working solution.
- Incubate the cells or seedlings in the dye solution so that the plasma membrane becomes stained in the pulse step.
- Wash the sample to remove the dye from the medium.
- Transfer the sample to fresh medium without dye so that the dye can move inward through endosomes in the chase step.
- Allow sufficient time for the dye to reach the tonoplast where it is finally deposited.
- Visualize the sample under fluorescence or confocal microscopy where the vacuolar membrane appears as a fluorescent ring.
C. Metabolic Vitality Staining: ViaVac™/FUN® 1 (Yeast)
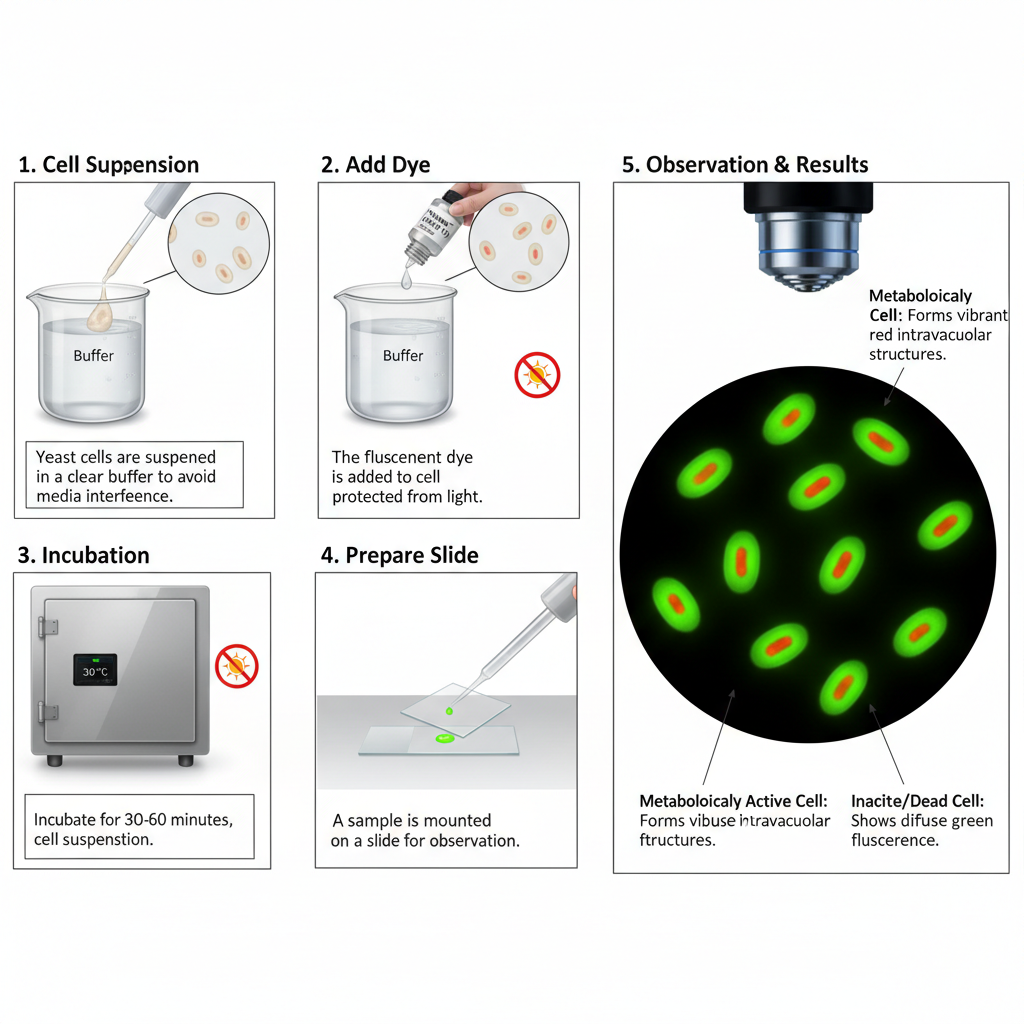
- Suspend the yeast cells in a clear buffer because rich media can interfere with the uptake of the stain.
- Add the ViaVac (FUN 1) dye to the buffer so that the final concentration lies within the required range.
- Incubate the stained cells for some minutes at room temperature or 30 °C and keep the sample protected from light.
- If a cell wall stain is needed, add it only after the ViaVac incubation so that dye uptake is not inhibited.
- Observe the cells under fluorescence microscopy where metabolically active cells form red intravacuolar structures and inactive cells show diffuse green fluorescence.
D. Specialized: Protozoan Contractile Vacuoles
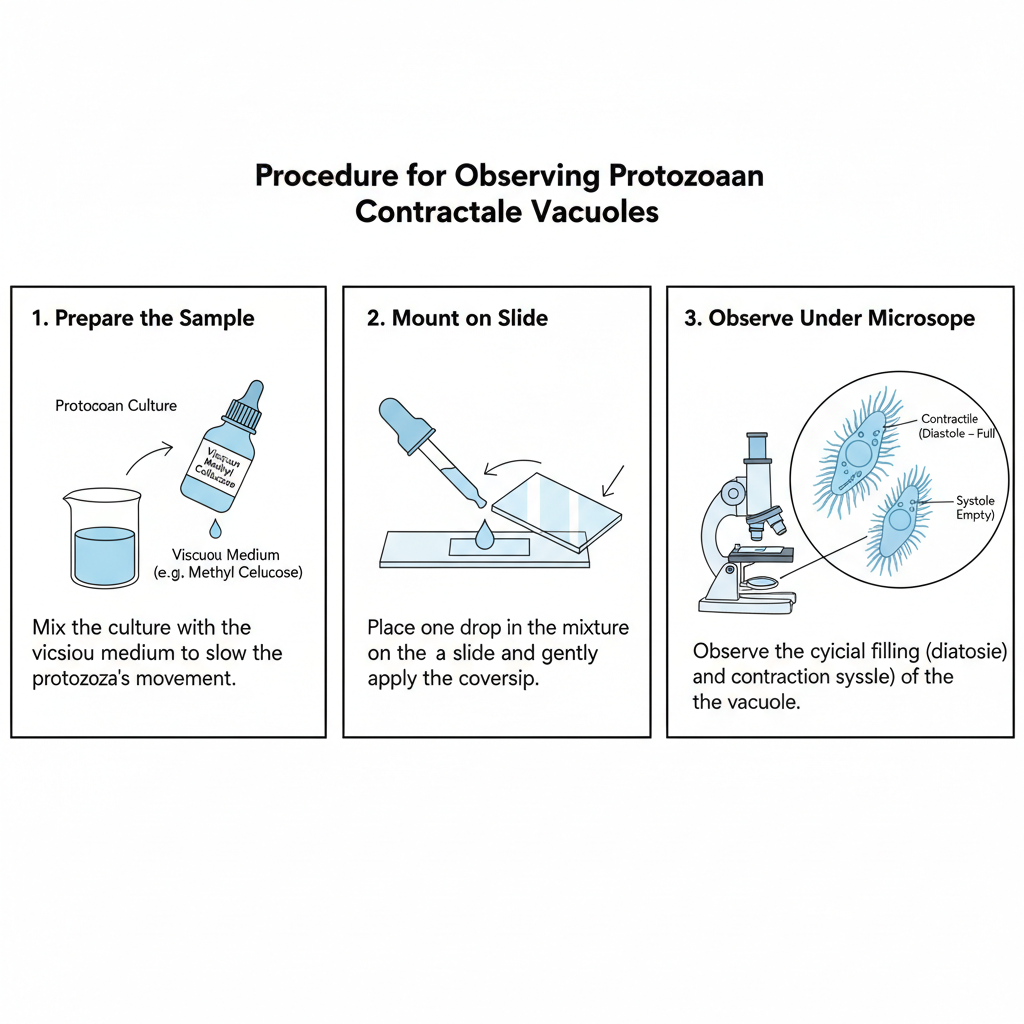
- Mix the protozoan culture with a viscous medium because this slows the movement of the organisms.
- Place the mixture on a slide and apply a coverslip gently for mounting.
- Observe the contractile vacuole under bright-field or phase-contrast microscopy.
- Video recording is often needed because the contraction and expansion cycle may not be visible in every moment.
E. Staining for Lumen pH: BCECF-AM (Plants)
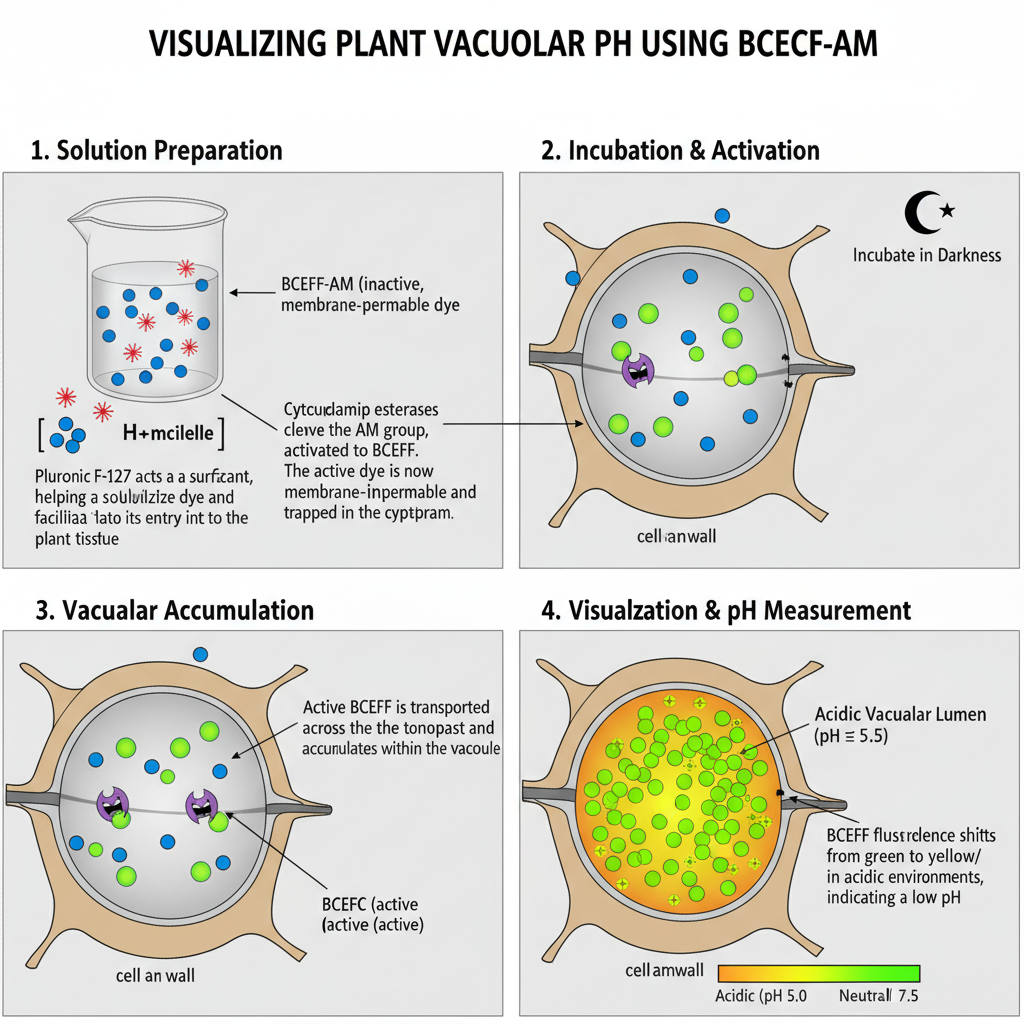
- Add Pluronic F-127 to the staining solution because it helps the dye to enter the plant tissues.
- Incubate the seedlings or cells in the BCECF-AM solution for the required time in darkness so that the dye can accumulate inside the vacuole.
- Wash the sample with liquid medium to remove the excess dye from the outer surfaces.
- Visualize the sample immediately with the proper excitation setting where the acidic vacuolar lumen appears green or orange.
Result of Vacuole Staining
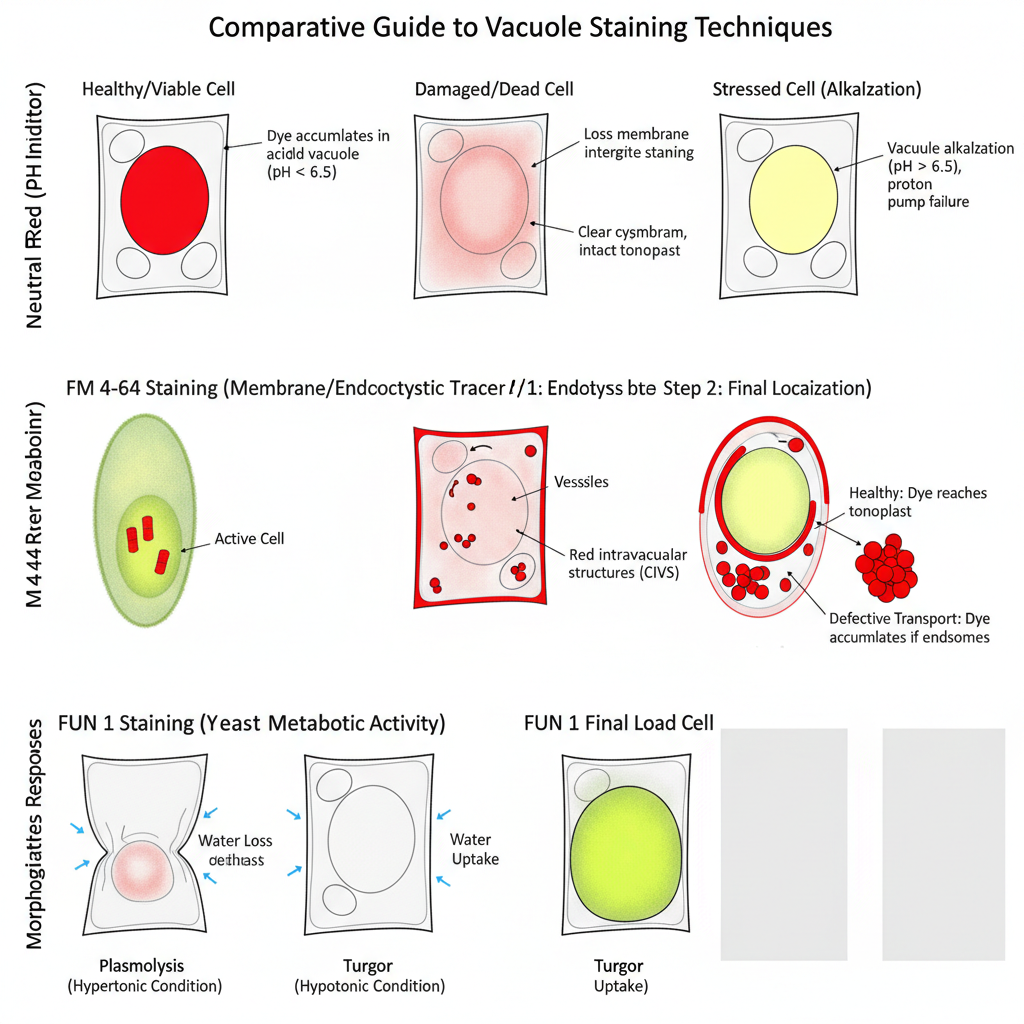
- In Neutral Red staining, a healthy cell shows deep red coloration restricted to the vacuolar lumen because the dye is trapped in the acidic environment.
- The cytoplasm remains clear in viable cells, indicating that the tonoplast is intact and the proton gradient is maintained.
- Diffuse red staining in the cytoplasm or staining of the nucleus indicates loss of membrane integrity, and the cell is considered dead or damaged.
- A change of colour from red to yellow shows alkalization of the vacuole which suggests failure of proton pumps or decline in cell viability.
- In FM 4-64 staining, the plasma membrane is labelled first and then small fluorescent punctate bodies appear as the dye moves inside.
- A clear fluorescent ring around the vacuole is formed at the end, showing proper integration of the dye into the tonoplast.
- If vesicular transport is defective, the dye accumulates in endosomal compartments rather than reaching the vacuole.
- In FUN 1 staining, metabolically active yeast cells produce red fluorescent structures inside the vacuole, showing active processing of the dye.
- Inactive or dead cells show diffuse green or yellow fluorescence without any intravacuolar structures.
- Vacuole staining also shows stress responses such as shrinking of the vacuole in plasmolysis, or turgid expansion in hypotonic conditions.
- In starved mammalian cells, large vacuoles may be formed, pushing the nucleus and cytoplasm aside.
- When fluorescent protein markers are used, proper accumulation inside the vacuole indicates correct trafficking, while mislocalization shows defective transport.
Summary Table of Vacuole Staining Results
| Staining Method | Result in Healthy/Active Cells | Result in Dead/Inactive or Defective Cells |
|---|---|---|
| Neutral Red | Deep red colour inside the vacuolar lumen due to ion trapping in acidic conditions. Cytoplasm stays clear. | Diffuse red staining of cytoplasm or nucleus. Yellow colour indicates alkaline vacuole or failure of proton pumps. |
| FM 4-64 (Endocytosis) | Plasma membrane labelled first, then small fluorescent puncta, and finally a clear fluorescent ring marking the tonoplast. | Dye accumulates in prevacuolar compartments when vesicular trafficking is defective and does not reach the vacuolar membrane. |
| ViaVac™ / FUN® 1 (Yeast) | Formation of red fluorescent intravacuolar tubular structures processed by active metabolism. | Diffuse green or yellow fluorescence throughout the cytoplasm with no red vacuolar structures. |
| Stress Responses (Plants/Other Cells) | Plasmolysis shows vacuole shrinkage in hypertonic solution; turgid expansion seen in hypotonic conditions. | In starvation, large vacuoles displace nucleus and cytoplasm. Mislocalization of GFP markers shows defective trafficking. |
Uses of Vacuole Staining
- It is used to assess the viability of cells because dyes like Neutral Red accumulate only in living cells maintaining an acidic vacuole.
- It is used to study metabolic activity in yeast where FUN 1 forms red intravacuolar structures only in metabolically active cells.
- It is used to trace membrane movement since FM 4-64 labels the plasma membrane first and then moves inward by endocytosis to reach the tonoplast.
- It helps in identifying transport mutants when the dye accumulates in intermediate compartments instead of the vacuolar membrane.
- It is used to observe fission and fusion events of vacuoles during growth and environmental changes.
- It is used to monitor physiological responses such as plasmolysis, where the vacuole shrinks in hypertonic conditions.
- It helps in observing intracellular pH because some dyes change colour or fluorescence according to acidity.
- It is used to study contractile vacuoles in protozoa where rhythmic contraction and expansion can be observed.
- It is used to track protein sorting pathways when fluorescent proteins accumulate inside the vacuole or on the tonoplast.
- It helps in distinguishing between soluble and membrane cargo during vacuolar transport.
- It is used to visualize stress-induced vacuolization seen in starved mammalian cells.
- It helps in identifying large vacuoles that displace nucleus and cytoplasm giving signet-ring like appearances.
Advantages of Vacuole Staining
- It helps in visualizing transparent vacuoles which are otherwise difficult to see in normal microscopy because staining clearly shows their size, shape, and position.
- It is used to observe morphological responses like plasmolysis and turgor changes in plant cells.
- It helps in identifying contractile vacuoles in protozoa where the organelle may become invisible during movement.
- It is used to detect stress-related large vacuoles in mammalian cells which sometimes produce signet ring-like appearance.
- It provides a reliable method for assessing cell viability because dyes like Neutral Red accumulate only in active and living cells.
- It can distinguish metabolically active yeast cells from inactive ones using FUN 1 staining.
- It helps in monitoring intracellular pH since some dyes act as pH indicators and change colour or fluorescence based on acidity.
- It allows early detection of physiological stress when the vacuolar pH becomes alkaline.
- It is used for tracing membrane movement and endocytosis because FM 4-64 follows a defined path from plasma membrane to vacuole.
- It helps in identifying mutants with defective trafficking pathways when the dye accumulates in intermediate compartments.
- It allows observation of fusion and fission events in the vacuole.
- It can differentiate between different types of vacuoles such as lytic and storage vacuoles when specific fluorescent markers are used.
- It helps in identifying the origin of vacuoles in mammalian cells through co-staining with lysosomal or autophagosomal markers.
- It is simple and cost-effective because Neutral Red staining requires basic solutions and simple bright-field microscopy.
- It is generally non-toxic for short-term observation of living tissues.
Limitations of Vacuole Staining
- Many stains depend on the physiological state of the cell, and staining can fail when the vacuole loses its acidic environment.
- Dyes like Neutral Red need an active proton gradient; when the vacuole becomes alkaline the dye cannot accumulate which may give false negative results.
- Some stains need metabolically active cells, and dyes like FUN 1 or FM 4-64 do not work in inactive or energy-depleted cells.
- Dead cells show dye leakage into the cytoplasm making it difficult to identify the vacuole clearly.
- Neutral Red can also stain cell walls, lignified tissues, or nuclei in fixed samples which can create confusion.
- FM 4-64 stains several intermediate compartments before reaching the tonoplast, and early observations can misidentify these structures.
- Neutral Red solutions may produce precipitates that appear as false cellular bodies.
- Lipophilic dyes sometimes alter membrane behavior which may affect normal membrane trafficking.
- Some dyes increase cell sensitivity to light, and long exposure can kill the sample due to phototoxic activity.
- Weak-base dyes may slowly increase vacuolar pH if kept too long which changes natural physiology.
- Protozoa move very quickly, and immobilization is needed for observing contractile vacuoles which adds extra steps.
- Some growth media interfere with dye uptake in yeast, and cells must be transferred to a different buffer.
- Fluorescent proteins can be degraded in the lytic vacuole which reduces the visible signal.
- Large stress-induced vacuoles in mammalian cells can resemble signet ring cells and need additional markers for correct interpretation.
- APExBIO. (n.d.). Neutral red staining solution protocol (Cat. No. K1185).
- Biotium. (2016, May 19). Yeast vitality staining kit: Product information.
- Dreier, A. (2025, August 1). Can dead cells have full membrane integrity? [Online forum post]. ResearchGate.
- Halpern, J. (2025, April 26). 4.8: Red onion osmosis lab. Biology LibreTexts.
- Kang, H., Kim, S. Y., Song, K., Sohn, E. J., Lee, Y., Lee, D. W., Hara-Nishimura, I., & Hwang, I. (2012). Trafficking of vacuolar proteins: The crucial role of Arabidopsis vacuolar protein sorting 29 in recycling vacuolar sorting receptor. The Plant Cell, 24(12), 5058–5073. https://doi.org/10.1105/tpc.112.103481
- Lee, Y., Jang, M., Song, K., Kang, H., Lee, M. H., Lee, D. W., Zouhar, J., Rojo, E., Sohn, E. J., & Hwang, I. (2013). Functional identification of sorting receptors involved in trafficking of soluble lytic vacuolar proteins in vegetative cells of Arabidopsis. Plant Physiology, 161(1), 121–133. https://doi.org/10.1104/pp.112.210914
- Liang, W., Wang, K., & Wang, J. (2008). A method for detecting algae cell activity by neutral red staining (Patent No. CN101201312A). Beijing Forestry University.
- Microscope World. (2024, February 11). How to prepare an onion cell slide.
- Phan Kiệt. (n.d.). Observation of plant cell. Scribd.
- Quality Biological. (n.d.). Neutral red solution (0.33%) for staining: Introduction components and recommended storage materials protocol.
- Scheuring, D., Schöller, M., Kleine-Vehn, J., & Löfke, C. (2015). Vacuolar staining methods in plant cells. Methods in Molecular Biology, 1242, 83–92. https://doi.org/10.1007/978-1-4939-1902-4_8
- Schwab, B., & Hülskamp, M. (2010). Neutral red staining for plant vacuoles. Cold Spring Harbor Protocols, 2010(6), pdb.prot4953. https://doi.org/10.1101/pdb.prot4953
- Stefano, G., Renna, L., & Brandizzi, F. (2017). Plant cell vacuoles: Staining and fluorescent probes (Technical Report). U.S. Department of Energy Office of Scientific and Technical Information.
- Stefano, G., Renna, L., & Brandizzi, F. (2018). Plant cell vacuoles: Staining and fluorescent probes. Methods in Molecular Biology, 1789, 55–63. https://doi.org/10.1007/978-1-4939-7856-4_5
- Sun, D., An, X., & Cheng, Y. (2025). The formation and features of massive vacuole induced by nutrient deficiency in human embryonic kidney cells. Frontiers in Bioscience (Landmark Edition), 30(1), 26796. https://doi.org/10.31083/FBL26796
- Thermo Fisher Scientific. (n.d.). Probes for lysosomes, peroxisomes and yeast vacuoles—Section 12.3. The Molecular Probes Handbook.
- Vida, T. A., & Emr, S. D. (1995). A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. The Journal of Cell Biology, 128(5), 779–792. https://doi.org/10.1083/jcb.128.5.779
- Witty, M. (2009). Methods for observing protozoan contractile vacuoles. American Society for Microbiology.
- Wudick, M. M., Luu, D. T., Tournaire-Roux, C., Sakr, S., & Maurel, C. (2014). Vegetative and sperm cell-specific aquaporins of Arabidopsis highlight the vacuolar equipment of pollen and contribute to plant reproduction. Plant Physiology, 164(4), 1697–1706.
- Yoshida, K., Ohnishi, M., Fukao, Y., Okazaki, Y., Fujiwara, M., Song, C., Nakanishi, Y., Saito, K., Shimmen, T., Suzaki, T., Hayashi, F., Fukaki, H., Maeshima, M., & Mimura, T. (2013). Studies on vacuolar membrane microdomains isolated from Arabidopsis suspension-cultured cells: Local distribution of vacuolar membrane proteins. Plant and Cell Physiology, 54(10), 1571–1584. https://doi.org/10.1093/pcp/pct107
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.