Transfection refers to the introduction of foreign DNA (non-host genome genetic material) into a cell. The primary goal of transfection is to modify the host genome in order to express or inhibit the production of the linked protein. The primary purpose of this page is to provide readers with a thorough explanation of the fundamental principle of transfection, as well as the distinctions between the various types of transfections and contemporary transfection techniques. It also examines the parameters that govern the transfection technique and its applications. These classifications are based on the stability of the transfection’s end result. Transfection can be classified as either transient or stable, depending on the stability of the transfection’s end product.
In transfection technology, there are a number of key terminology that are regularly used; the following list summarises some of them.
- Transformation: Transformation is the term used to define and refer to the nonviral technique of gene transfer. A bacterial or eukaryotic animal cell may be the host or recipient of such genetic material.
- Transduction: It is also referred to as the transduction infection. It is used to describe the transmission of genetic material from a virus to the recipient.
- Stable Transfection: The transfection in which the genetic material of interest is integrated into the genome of the host.
- Transient Transfection: The circumstance in which genetic material is not incorporated into the genome of the host cell.
- Transfection: Transfection is the process of nonviral gene delivery into recipient cells. DNA, RNA, messenger RNA (mRNA), small interfering RNA (siRNA), microRNA (miRNA), and short hairpin RNA are examples of exogenous nucleic acid material (shRNA).
History and Development
Calcium phosphate transfection was one of the early chemical-based approaches discovered to transfer exogenous nucleic acids to mammalian cells in culture. This approach relies on a saline solution containing calcium chloride and HEPES buffer to transfer plasmid DNA. The adaption of lipid- and polymer-based carrier molecules capable of binding nucleic acids led to their use in transfection. Numerous of these chemicals generate liposomes, which can merge with the cellular membrane to transport RNA or DNA to the cell. This method is useful for overcoming the natural resistance of cells to the insertion of genes while retaining the safety of the organisms used. During this procedure, the injected nucleic acid may be transitory (does not replicate) or stable and fused into the recipient’s genome, thereby replicating when the host genome replicates. This is a crucial distinction to comprehend when dealing with transfection, as the ultimate result is variable and must be determined based on the objective of a particular transfection experiment.
In the world of biomedical research, the phrases “transfection” and “transformation” have had varying meanings over time. In recent years, transfection has come to encompass any artificial introduction of foreign nucleic acid into a cell. Recent developments in delivery methods have permitted the introduction of a new generation of transfection reagents for both in vitro and in vivo applications. In basic life sciences research and linked clinical trials, these methodologies are a powerful tool.
What is transfection?
- Transfection is the method of introducing nucleic acids into eukaryotic cells on purpose. It may also refer to additional techniques and cell types, although the following phrases are typically preferred: Transformation is commonly used to describe the non-viral transfer of DNA between bacteria and non-animal eukaryotic cells, including plant cells. In animal cells, the appropriate term is transfection, as transformation is also used to describe progression to a malignant state (carcinogenesis) in these cells. Transduction is frequently used to describe the transfer of genes into eukaryotic cells by means of a virus.
- Trans- and infection combine to form the word transfection. It is possible to transfect genetic material (such as supercoiled plasmid DNA or siRNA constructs).
- Typically, transfection of animal cells includes the temporary opening of pores or “holes” in the cell membrane to permit the uptake of material.
- Calcium phosphate (i.e. tricalcium phosphate), electroporation, cell squeezing, or mixing a cationic lipid with the material to generate liposomes that fuse with the cell membrane and deposit their cargo within are all viable methods for transfection.
- Target cells may exhibit unexpected morphologies and abnormalities after transfection.
- The term’s meaning has evolved. The original definition of transfection was “infection through transformation,” or the introduction of DNA or RNA from a virus or bacteriophage that infects prokaryotes into cells, resulting in an infection.
- For bacterial and archaeal cell research, transfection preserves its original definition as a subset of transformation.
- Because the term transformation had another meaning in animal cell biology (a genetic change allowing long-term propagation in culture or the acquisition of characteristics typical of cancer cells), the term transfection acquired, for animal cells, its current meaning of a change in cell properties caused by DNA introduction.
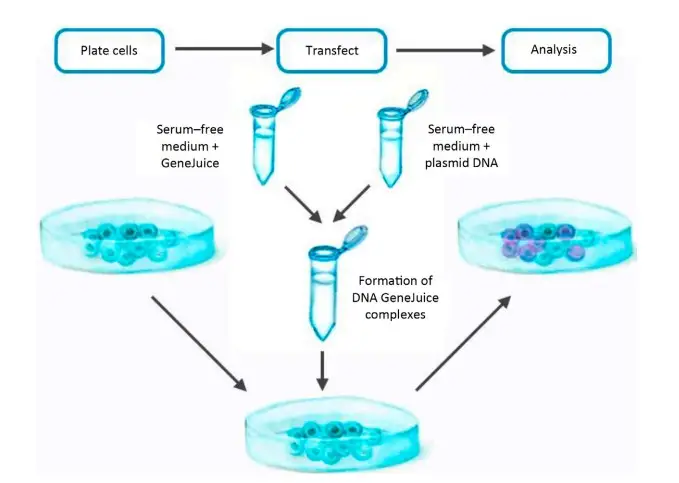
Type of Molecule Transfected
Commonly, plasmid DNA is transfected into cells, although other macromolecules can also be delivered. Transfection techniques have been used successfully to deliver DNA oligonucleotides, short interfering RNA (siRNA), proteins, and ribonucleoprotein complexes (RNPs) into cells, for instance. However, the optimal conditions for plasmid DNA transfer will likely need to be adjusted for the transfer of other macromolecules. The transfected agent must always be of excellent quality and relative purity. Nucleic acids should be devoid of proteins, other nucleic acid contaminants, and chemicals (e.g., salts from oligo synthesis). Protein should be pure and dissolved in a solvent that does not harm cell health.
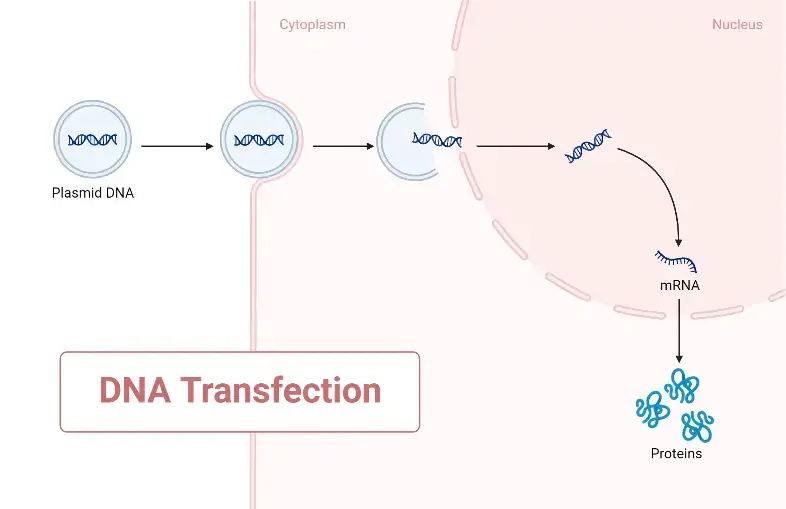
Transient Versus Stable Transfection
- Transient transfection can be described as a technique of transfection in which exogenous material is not incorporated into the host genome; moreover, mRNA transfection can be termed transient transfection. The reason it is mRNA is because of the coding elements of the cell, which undergo translation to produce the protein. When mRNA is injected into a cell, it expresses the protein it codes for and then degrades due to its relatively short half-life. Therefore, in both instances, the exogenous material undergoes destruction and is categorised as transient transfection.
- The most common method is stable transfection, which involves the insertion of external genetic material into the host genome. This approach ensures the uninterrupted expression of the protein encoded by the foreign material. These typically result in lasting changes to the host’s genome. Typically, the external genetic material is transported directly into the nucleus of the host.
| Transient Transfection | Stable Transfection |
| Chemical or physical methods | Biological or physical |
| Short-term expression | Long-term expression with persistent gain-of-function or loss-of-function |
| No genomic integration | Risk of nonspecific integration |
Principle of Transfection
According to the definition of transfection, the external genetic material must enter the cell via the cell membrane. It is essential to highlight that the genetic material, whether DNA or RNA, is negatively charged as a result of the proteins that surround it. Therefore, in an unmanipulated environment, exogenous DNA cannot cross the cell membrane. However, during transfection, the genetic material becomes coupled with positively charged molecules or is inserted straight into the nucleus of the host cell. This results in the elimination of the barrier for traversing the charged membrane. Chemical transfection and physical transfection can be distinguished according to the method employed to transmit exogenous genetic material, namely conjugation with cationic chemicals or insertion into the nucleus. As an example of this category, transduction falls under biological transfection, in which transfection is mediated by viral vectors.
Transfection Methods (Types of Transfection)
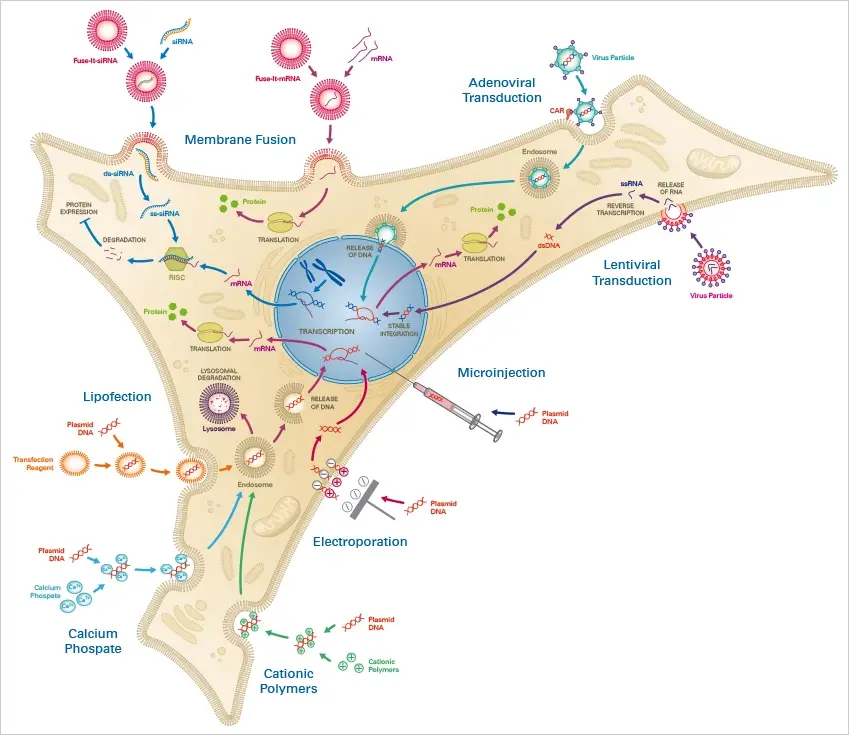
Without external stimulus, cells often do not take up foreign nucleic acids. Thus, an efficient and biocompatible method of transfection is required, which must be precisely matched to the cell type and the substance to be transported. Numerous transfection methods have been created to date, each with its own advantages and limitations for its respective application area.
Three distinct categories of transfection techniques exist:
- Chemical transfection
- Physical transfection
- Viral transduction (Biological transfection).
A. Chemical Methods of transfection
Chemical transfection is a method of introducing foreign DNA into cells by using chemicals to increase the permeability of the cell membrane. The most common chemicals used are liposomes, polyethylenimine (PEI) and calcium phosphate. This method is simple, fast, and efficient, but it can also be less stable than other transfection methods and can have significant toxicity. The choice of chemical reagent and the conditions used can greatly affect the efficiency and specificity of transfection.
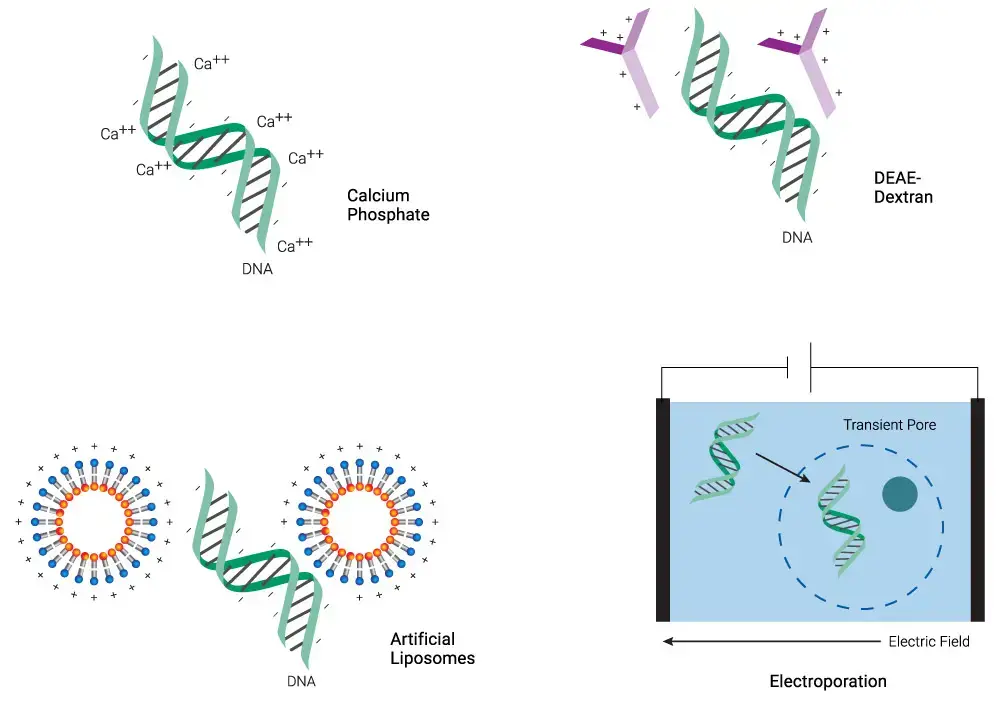
List of Chemical Methods of transfection;
- Membrane Fusion
- Lipofection
- Calcium Phosphate
- Cationic Polymers
1. Membrane Fusion
Membrane fusion is a process by which two separate lipid bilayers come together to form a single bilayer. This process is crucial for a number of biological processes such as exocytosis, endocytosis, and viral entry. During membrane fusion, the lipid bilayers become fluid and merge, creating a fusion pore through which the contents of one membrane can mix with the other. The process of membrane fusion is regulated by specific fusion proteins that initiate and control the formation and stability of the fusion pore. Membrane fusion can also be artificially triggered using chemical agents or physical stimuli, which can be useful for various research and biotechnological applications.
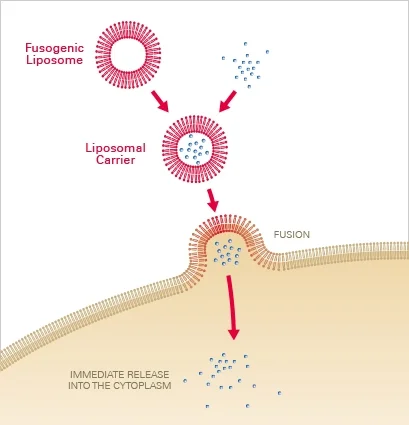
Principle
Liposomal carriers composed of neutral and cationic lipids are utilised to deliver the chemical of interest into the cell via membrane fusion. The molecules are first encapsulated in liposomal carriers, which, upon contact with the cell membrane, fuse instantaneously. The contained molecules are subsequently released directly into the cytoplasm, bypassing endocytosis and lysosomal destruction. Depending on the type of cell, this results in fusion efficiencies of 80–100% within seconds to a few minutes.
Applications
Membrane fusion is a revolutionary and vastly improved approach for transfecting molecules and particles into human cells. This novel approach can transfect not just cells that undergo cell division, but also primary and non-dividing cells. It enables the transfer of mRNA, siRNA, proteins, beads, and other compounds in a highly effective manner. Due to the fact that the reagents are non-toxic to sensitive and difficult-to-transfect cells, primary neurons, keratinocytes, and stem cells retain good viability following transfection. In addition, the extremely brief incubation periods reduce cell stress and produce an almost unaffected cell behaviour after molecular transfer.
2. Lipofection
Lipofection is a method of transfecting cells with genetic material (such as DNA or RNA) using lipid-based delivery systems. The lipids used in lipofection form stable complexes with the genetic material and can penetrate the cell membrane to deliver the genetic material into the cell. Lipofection has advantages over other transfection methods such as high efficiency, ability to transfect a wide range of cell types, and low toxicity. It is widely used in molecular biology and biotechnology for various applications, including gene expression analysis, gene therapy, and vaccine production.
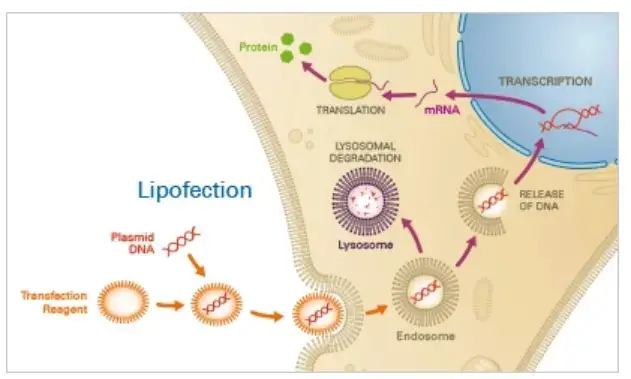
Principle
Typically, a mixture of neutral and cationic liposomes form complexes with the target nucleotides. Through endocytosis, these complexes traverse the cell membrane and subsequently release the nucleotides into the cytoplasm. The nucleotides then either effectively escape the endosome or suffer lysosomal degradation, with lysosomal degradation potentially resulting in decreased transfection efficacy. After escaping endosomes, the nucleotide is expressed in target cells.
Applications
Lipofection is frequently employed to transport nucleic acids such as RNA and DNA into eukaryotic cells. After protocol improvement, this approach is simple to implement and produces highly reproducible transient and stable transfection results. Nonetheless, lipofection efficiency is highly dependent on cell type and must be examined and optimised beforehand. Due to the high cellular sensitivity, the transfection procedure may reduce the viability of cells, particularly primary and non-dividing cells. In contrast to membrane fusion, lipofection relies on endosomal molecule absorption, which may lengthen the time necessary for analysis.
3. Calcium Phosphate
Calcium Phosphate transfection is a method of delivering DNA or RNA into cells using a mixture of calcium phosphate and the genetic material. This method is based on the ability of calcium to cause the formation of calcium phosphate precipitates that can complex with the genetic material and enter the cells. Calcium Phosphate transfection is a simple, inexpensive, and versatile method of transfecting cells, especially in vitro. However, it has some limitations, including low transfection efficiency and toxicity to some cell types. Calcium Phosphate transfection is widely used in molecular biology and biotechnology for various applications, including gene expression analysis, gene therapy, and vaccine production.
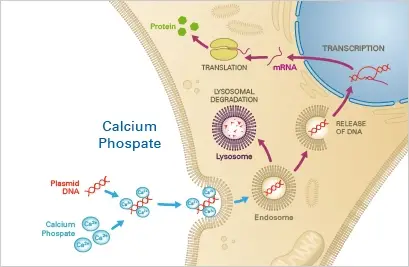
Principle
A precipitate composed of nucleotides, calcium, and phosphate buffer is taken up by the cells via endocytosis. The nucleotides then either escape the endosome or undergo lysosomal degradation, the latter of which may result in a decrease in transfection efficacy. The escaping nucleotides are then able to be expressed in the target cells.
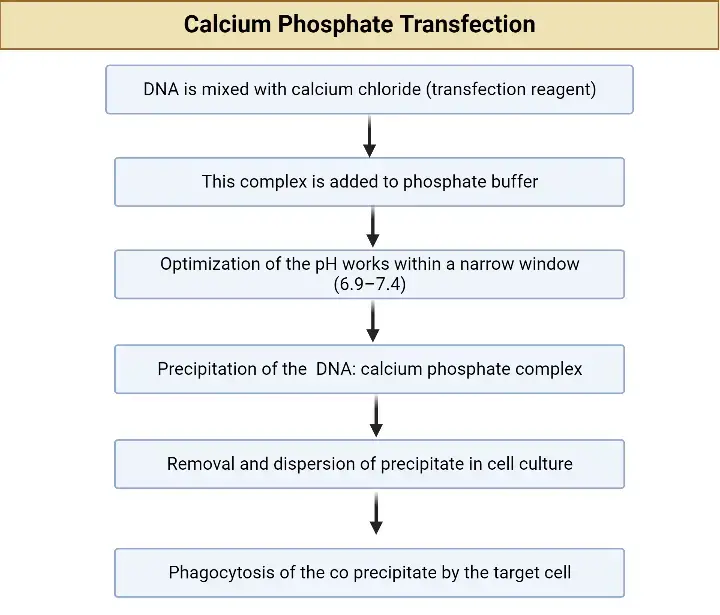
Applications
Typically, a mixture of neutral and cationic liposomes form complexes with the target nucleotides. Through endocytosis, these complexes traverse the cell membrane and subsequently release the nucleotides into the cytoplasm. The nucleotides then either effectively escape the endosome or suffer lysosomal degradation, with lysosomal degradation potentially resulting in decreased transfection efficacy. After escaping endosomes, the nucleotide is expressed in target cells.
4. Cationic Polymers
Cationic polymers are synthetic polymers with positive charges that can be used for transfection of cells with genetic material. Cationic polymers can form complexes with negatively charged DNA or RNA and facilitate their uptake into cells. This method of transfection is often referred to as polyfection. Cationic polymers are attractive for transfection because they can efficiently deliver large amounts of genetic material into cells with low toxicity. Additionally, they can be easily modified to optimize transfection efficiency and specificity. Cationic polymers are widely used in molecular biology and biotechnology for various applications, including gene expression analysis, gene therapy, and vaccine production.
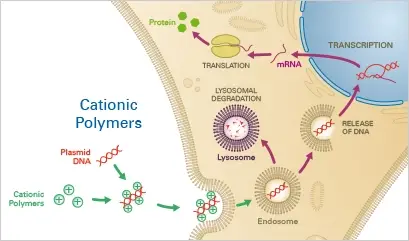
Principle
The negatively charged nucleotide backbones combine with cationic polymers, such as diethylaminoethyl (DEAE)-dextran, to create complexes. The complex is then mostly endocytosed into the cells. If lysosomal degradation does not occur, the nucleotide can escape from the endosome and enter the cytoplasm of the host cell, resulting in the expression of the transgene.
Applications
Nucleotides can be transiently transfected into eukaryotic cells in an affordable and straightforward manner using cationic polymers. After protocol optimization, it is simple to duplicate the results. As a disadvantage, cationic polymers have a very low transfection effectiveness (10%) in a variety of cell types, including primordial cells. Moreover, because cationic polymers are extremely cytotoxic, they are unsuitable for transfection of sensitive cells and the establishment of stable cell lines.
B. Physical Methods of Transfection
Physical approaches are conceptually the simplest, employing physical means to drive transfected material into the nucleus of the target cell. Electroporation is the most common physical approach, in which short electrical pulses rupture the cell membrane and allow the transfected nucleic acids to enter the cell. Other physical approaches employ alternative mechanisms to puncture the cell membrane: Sonoporation employs high-intensity ultrasound (attributed mostly to the cavitation of gas bubbles interacting with adjacent cell membranes), whereas optical transfection employs a highly focused laser to create a ~1 µm-diameter hole.
Several methods force the nucleic acid into the cell, including microinjection of nucleic acid with a fine needle, biolistic particle delivery, in which nucleic acid is attached to heavy metal particles (typically gold) and propelled into the cells at high speed, and magnetofection, in which nucleic acids are attached to magnetic iron oxide particles and driven into the target cells by magnets.
Hydrodynamic delivery is a technique utilised in mice and rats in which nucleic acids can be delivered to the liver by injecting a reasonably significant amount of blood in less than 10 seconds; almost all of the DNA is expressed in the liver using this method.
List of Physical Methods of Transfection
- Microinjection
- Electroporation
- laser-mediated transfection
- Biolistic particle delivery
- Magnetic-assisted transfection
- Sonoporation
1. Microinjection
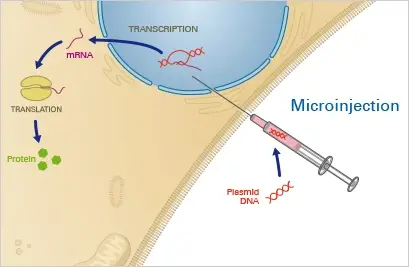
Microinjection is a physical method of transfection where a small volume of DNA solution is injected directly into the cytoplasm of a cell using a fine needle. This method is often used to introduce DNA into cells that are difficult to transfect using other methods, such as primary cells, embryonic cells, and stem cells.
Microinjection is a highly precise and targeted method of transfection, and it allows for the introduction of large amounts of DNA into a single cell. However, it is also a technically demanding method and requires specialized equipment and training to perform.
Microinjection is widely used in cellular and molecular biology, as well as in genetic engineering, to study gene function, produce transgenic animals, and develop new therapies for genetic diseases.
Principle
The target cell is positioned under a microscope and fastened with a pipette using this physical method. Using a fine glass capillary needle, the nucleotide solution is then directly injected into the cytoplasm and/or the nucleus. This precise method necessitates a somewhat costly microinjection device and considerable expertise and practise. Once the nucleotide has entered the nucleus, it can instantly integrate into the native DNA. Given these conditions, microinjection is highly efficient and permits the transfer of precisely controlled nucleotide concentrations. Nevertheless, because each single cell must be microinjected manually, this procedure is extremely time-consuming.
Applications
Microinjection permits the efficient transport of regulated amounts of nucleotides into the nucleus of a particular target cell. It is a very precise, but time-consuming, expensive, and low-throughput transfection approach. Microinjection is typically reserved for specialised purposes, such as the manipulation of single cells or the creation of transgenic animals, as in the case of pronuclear injection in mice.
2. Electroporation
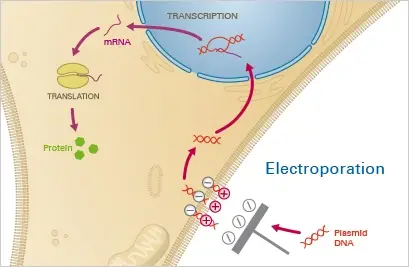
Electroporation is a physical method of transfection that uses an electric field to create transient pores in the cell membrane, allowing DNA to enter the cell. The procedure involves exposing cells to a high-voltage electrical pulse, which causes the cell membrane to temporarily become permeable, allowing the DNA to enter.
Electroporation is a widely used and efficient method of transfection, as it allows for the introduction of large amounts of DNA into cells in a relatively short amount of time. It is also a versatile method that can be used with a variety of cell types, including bacterial, yeast, and mammalian cells.
However, electroporation can also cause damage to the cells, so it is important to carefully control the parameters of the electrical pulse, such as voltage, duration, and pulse number, to minimize damage.
Electroporation is widely used in cellular and molecular biology, as well as in genetic engineering, to study gene function, produce transgenic animals, and develop new therapies for genetic diseases.
Principle
A mixture of cells and the nucleotide of interest is subjected to a strong electric field during electroporation. This causes temporary instability of the cell membrane, rendering it permeable to the nucleotides contained in the surrounding fluid. A brief electric pulse temporarily permeates the cell membrane, allowing the nucleotides contained in the surrounding fluid to enter the cytoplasm. After the electric field is removed, the cell membrane stabilises and encloses the nucleotides in the cytoplasm, where they are expressed.
Applications
Any type of cell can be transiently and permanently transfected using electroporation. This approach is simple and dependable, however it requires a large number of cells due to high cell death rates. Therefore, electroporation is inappropriate for delicate and challenging-to-cultivate cell types, such as primary cells. In addition, an expensive, specialised electroporation instrument is required.
3. laser-mediated transfection
Laser-mediated transfection, also known as laserfection or optoporation, involves the employment of a pulse laser to temporarily permeabilize cells in order to transfer nucleic acid material. This approach can be quite effective, and it has the added benefit of allowing the transfection of very small cells. Due to the need for a costly laser-microscope system, however, its application may be limited.
- Laser-mediated transfection is a physical method of transfection that uses laser light to introduce DNA into cells. The procedure involves exposing cells to laser light, which creates transient pores in the cell membrane, allowing the DNA to enter. The laser light can be delivered either directly to the cells or to a photosensitizing agent that is taken up by the cells, which then produces the pores in response to the laser light.
- Laser-mediated transfection is a highly efficient and specific method of transfection, as the laser light can be precisely targeted to specific cells or regions of the tissue. It is also a relatively non-invasive method, as it does not require the use of electric fields or chemicals.
- However, laser-mediated transfection can be technically demanding, and it requires specialized equipment, including a laser source and optical components. It can also cause damage to the cells, so it is important to carefully control the parameters of the laser exposure, such as laser intensity and duration.
- Laser-mediated transfection is widely used in cellular and molecular biology, as well as in genetic engineering, to study gene function, produce transgenic animals, and develop new therapies for genetic diseases.
4. Biolistic particle delivery
Biolistic particle delivery employs gold particles coated with nucleic acid and a high velocity gene gun to deliver these particles into cells. The approach is dependable and applicable to a large variety of cell types, although it requires specialist equipment and physically damages samples.
- Biolistic particle delivery, also known as ballistic transfection or gene gun transfection, is a physical method of transfection that uses microscopic particles, such as gold or tungsten, to deliver DNA into cells. The procedure involves coating microscopic particles with DNA and physically shooting them into the cells using a special device called a gene gun. The particles penetrate the cell membrane and deliver the DNA into the cytoplasm, where it can be expressed.
- Biolistic particle delivery is a highly efficient method of transfection, and it has been shown to be effective with a variety of cell types, including plant, animal, and bacterial cells. It is also a versatile method, as it can be used for a range of applications, including the production of transgenic plants, the study of gene function, and the development of new therapies for genetic diseases.
- However, biolistic particle delivery can also be technically demanding, and it can cause damage to the cells, so it is important to carefully control the parameters of the particle delivery, such as particle size, velocity, and pressure.
- Biolistic particle delivery is widely used in cellular and molecular biology, as well as in genetic engineering, to study gene function, produce transgenic animals and plants, and develop new therapies for genetic diseases.
5. Magnetic-assisted transfection
- Magnetic-assisted transfection (also known as magnetic nanoparticle-mediated transfection) is a physical method of transfection that uses magnetic particles to deliver DNA into cells. The procedure involves coating magnetic particles with DNA, and then exposing the cells to a magnetic field, which attracts the magnetic particles to the cell surface. The particles then enter the cell and release the DNA, allowing it to be expressed.
- Magnetic-assisted transfection is a relatively non-invasive and gentle method of transfection, and it has been shown to be effective with a variety of cell types, including primary cells and stem cells. It also has the advantage of being scalable, making it suitable for high-throughput transfection applications.
- However, magnetic-assisted transfection can be expensive and requires specialized equipment, including a magnetic field generator and magnetic particles.
- Magnetic-assisted transfection is widely used in cellular and molecular biology, as well as in genetic engineering, to study gene function, produce transgenic animals, and develop new therapies for genetic diseases.
6. Sonoporation
This method uses high-frequency sound waves to create transient pores in the cell membrane, allowing DNA to enter the cell.
- Sonoporation is a physical method of transfection that uses high-frequency sound waves (ultrasound) to create transient pores in the cell membrane, allowing DNA to enter the cell. The procedure involves exposing cells to focused ultrasound waves, which cause mechanical stress on the cell membrane, creating small pores that allow the DNA to enter.
- Sonoporation is a relatively non-invasive and gentle method of transfection, and it has been shown to be effective with a variety of cell types, including primary cells, stem cells, and cancer cells. It is also a scalable method, making it suitable for high-throughput transfection applications.
- However, sonoporation can be technically demanding, and the parameters of the ultrasound exposure, such as frequency, intensity, and duration, need to be carefully controlled to minimize damage to the cells.
- Sonoporation is widely used in cellular and molecular biology, as well as in genetic engineering, to study gene function, produce transgenic animals, and develop new therapies for genetic diseases.
C. Biological Methods of Transfection
The term “transduction” is used to describe the transfer of nucleic acids into cells mediated by a virus. In contrast to the transfection of cells with foreign DNA or RNA, no transfection reagent is necessary in this instance. The viral vector, also known as the virion, is capable of independently infecting cells and transporting the DNA into the nucleus. After DNA is released into the nucleus, the protein of interest is synthesised by the cell’s own machinery.
Method Comparison
| Adenoviral transduction | Lentiviral transduction | Transfection | |
| Viral genome material | dsDNA | RNA | – |
| Transgene expression | Transient | Stable | Transient or stable |
| Genome integration | Episomal | Integration into host genome | Dependent on transfection method |
| Cell types | Dividing and non-dividing cells | Dividing and non-dividing cells | Dividing and non-dividing cells, dependent on transfection method |
| Efficiency | Up to 100% transduction efficiency | Up to 100% transduction efficiency | Variable transfection efficiency |
| Biosafety level | 2 | 2 | 1 |
List of Biological Methods of Transfection;
Some common biological methods of transfection include:
- Bacterial transfection: Bacteria such as E. coli can be used to deliver DNA into cells. The procedure involves transforming bacteria with the DNA of interest, and then exposing cells to the transformed bacteria.
- Viral transfection: Viruses, such as adenoviruses or lentiviruses, can be engineered to carry DNA into cells. The procedure involves exposing cells to the modified viruses, which then infect the cells and deliver the DNA.
- Liposomal transfection: Liposomes, which are spherical structures made of lipid bilayers, can be used to deliver DNA into cells. The procedure involves encapsulating the DNA into the liposomes, and then exposing the cells to the liposomes, which then fuse with the cell membrane and deliver the DNA into the cell.
These biological methods of transfection are often more physiological and less disruptive to the cells than physical methods, and they are widely used in cellular and molecular biology, as well as in genetic engineering, to study gene function, produce transgenic animals, and develop new therapies for genetic diseases.
However, biological methods of transfection can also have drawbacks, including the potential for immune response, toxicity, and the need for specialized equipment and expertise. Additionally, some methods may not be suitable for all cell types or may be limited in terms of the efficiency and specificity of DNA delivery.
1. Adenoviral Transduction
Adenoviral transduction is a method of delivering DNA into cells using adenoviruses as vectors. Adenoviruses are small, non-enveloped DNA viruses that can be engineered to carry foreign DNA into cells. The procedure involves exposing cells to adenoviruses that have been modified to carry the DNA of interest. The adenoviruses infect the cells, and the DNA is expressed, allowing the cells to produce the encoded protein.
Adenoviral transduction is a highly efficient and versatile method of transfection, and it has been shown to be effective with a variety of cell types, including primary cells, stem cells, and cancer cells. It is also a scalable method, making it suitable for high-throughput transfection applications.
However, adenoviral transduction can also have drawbacks, including the potential for immune response and toxicity, and the need for specialized equipment and expertise. Additionally, some cell types may be resistant to adenoviral infection, limiting the usefulness of this method.
Adenoviral transduction is widely used in cellular and molecular biology, as well as in genetic engineering, to study gene function, produce transgenic animals, and develop new therapies for genetic diseases. It is also used in gene therapy, as adenoviruses can be used to deliver therapeutic genes directly to cells in the body.
Principle
Adenoviruses are a type of DNA virus that efficiently delivers nucleotides to target cells. The virus enters the host cell via endocytosis after initial binding to the Coxsackievirus and adenovirus receptor (CAR), which is expressed on the cell membrane. After escaping endosomes, the viral genome is carried into the nucleus, where it is expressed by the host cell’s replication machinery. In contrast to the DNA of other viruses, adenoviral DNA is episomal, meaning it is not integrated into the host genome. Due of their great efficacy and minimal pathogenicity, replication-deficient adenoviruses are commonly utilised for transduction and gene therapy today.
Applications
Numerous eukaryotic cell types, including as human and rodent cells, have proved that adenoviral vectors are an effective transduction technique. This approach provides access to difficult-to-transfect cells, such as primary cells, in addition to dividing cell lines. Adenovirus-mediated transduction is always transitory, meaning that there is no integration of nucleotides into the host genome. Achieving conversion efficiency of up to 100 percent is simple. Importantly, level S2 biosafety is required for adenoviral transduction.
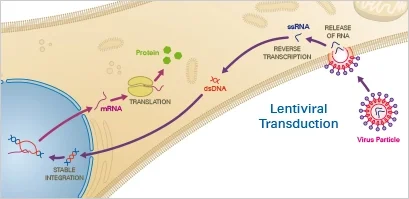
2. Lentiviral Transduction
Lentiviral transduction is a method of delivering DNA into cells using lentiviruses as vectors. Lentiviruses are a type of retrovirus that can infect both dividing and non-dividing cells, making them useful for a wide range of applications. The procedure involves exposing cells to lentiviruses that have been modified to carry the DNA of interest. The lentiviruses infect the cells, and the DNA is integrated into the cell’s genome, allowing the cells to stably express the encoded protein.
Lentiviral transduction is a highly efficient and versatile method of transfection, and it has been shown to be effective with a variety of cell types, including primary cells, stem cells, and cancer cells. It is also a scalable method, making it suitable for high-throughput transfection applications.
However, lentiviral transduction can also have drawbacks, including the potential for immune response and toxicity, and the need for specialized equipment and expertise. Additionally, the integration of the DNA into the cell’s genome can carry the risk of insertional mutagenesis, leading to unintended consequences.
Lentiviral transduction is widely used in cellular and molecular biology, as well as in genetic engineering, to study gene function, produce transgenic animals, and develop new therapies for genetic diseases. It is also used in gene therapy, as lentiviruses can be used to deliver therapeutic genes directly to cells in the body.
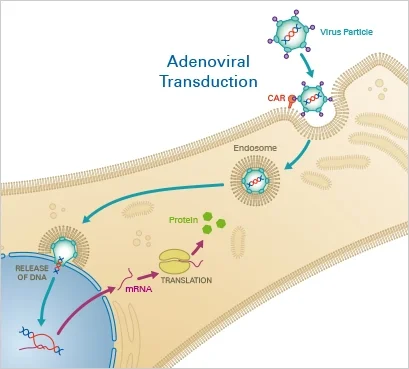
Principle
Lentiviruses, a subclass of retroviruses, have the capacity to integrate themselves permanently into the host cell’s genome. After the virus has entered the cell, the reverse transcriptase transcribes the viral RNA into double-stranded DNA, which then enters the nucleus. Finally, lentiviral integrase enzymes incorporate the transgene into the host DNA. When utilising lentiviral transduction, the user must consider the impact of genomic integration.
Applications
Recombinant lentiviral vectors are effective instruments for gene transfer with distinct benefits over other vectors: In addition to cells that undergo mitosis, they can also transduce non-dividing cells. In addition, lentiviruses permit stable gene transfer in vitro and in vivo because they integrate into the genome of the host cell and permit positive cell selection. In addition to primary neurons, lymphocytes, and macrophages, their host cell range is quite extensive. In addition, lentiviral vectors have demonstrated efficacy in transducing the brain, liver, muscle, and retina in vivo without causing damage or immunological reactions. Level S2 biosafety is required for lentiviral transduction.
3. Bacterial transfection
- Bacterial transfection is a method of delivering DNA into cells using bacteria as vectors. Bacteria such as Escherichia coli (E. coli) can be used to deliver DNA into cells by transforming the bacteria with the DNA of interest, and then exposing cells to the transformed bacteria. The bacteria can deliver the DNA into the cells either by conjugation, where the bacteria directly transfer the DNA to the cells, or by transduction, where the bacteria act as vectors to deliver the DNA.
- Bacterial transfection is a simple and affordable method of transfection that is widely used in molecular biology and genetic engineering. It is particularly useful for producing large amounts of recombinant proteins, as E. coli is a fast-growing organism and can produce large quantities of recombinant proteins.
- However, bacterial transfection can also have drawbacks, including the potential for toxicity and the need for specialized equipment and expertise. Additionally, some cell types may be resistant to bacterial infection, limiting the usefulness of this method.
- In conclusion, bacterial transfection is a powerful tool for delivering DNA into cells, but its applications are limited by its specific advantages and disadvantages, and it should be used in the context of the specific requirements of the experiment.
4. Liposomal transfection
- Liposomal transfection is a method of delivering DNA into cells using liposomes as vectors. Liposomes are spherical structures composed of a lipid bilayer that can encapsulate and deliver DNA into cells. The procedure involves encapsulating the DNA of interest into the liposomes, and then exposing the cells to the liposomes. The liposomes then fuse with the cell membrane and deliver the DNA into the cell.
- Liposomal transfection is a highly efficient method of transfection that is well-tolerated by cells, and it is widely used in cellular and molecular biology, as well as in genetic engineering, to study gene function, produce transgenic animals, and develop new therapies for genetic diseases.
- Liposomal transfection has several advantages, including the ability to deliver DNA into a variety of cell types, high efficiency of DNA delivery, and low toxicity to the cells. It is also a scalable method, making it suitable for high-throughput transfection applications.
- However, liposomal transfection can also have drawbacks, including the potential for variability in transfection efficiency and the need for specialized equipment and expertise. Additionally, the size and composition of the liposomes can affect the efficiency and specificity of DNA delivery, making it important to optimize these parameters for each specific application.
- In conclusion, liposomal transfection is a powerful tool for delivering DNA into cells, and it is widely used in a variety of applications in cellular and molecular biology, as well as in genetic engineering and gene therapy.
Generalized Protocol of Transfection
Comparative protocols for calcium phosphate transfections and lipofection procedures are provided here. Refer to product-specific protocols for specific instructions.
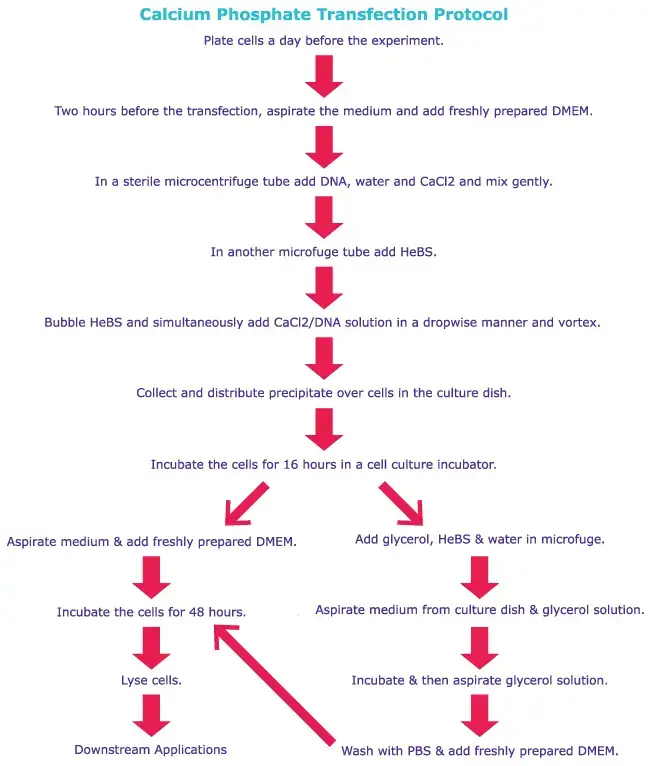
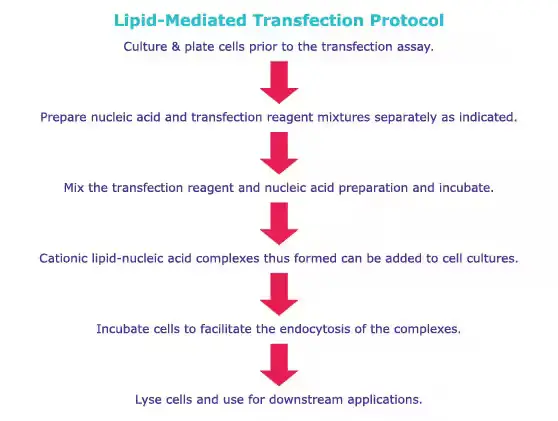
Overview of Advantages and Disadvantages of Different Transfection Methods
| Class | Method | Pros | Cons |
| Chemical | ● Cationic polymer ● Cationic lipid ● Calcium phosphate | – Relative ease of use – Relatively inexpensive | – Variable transfection efficiency – Toxicity to cells by some chemical compounds – May be difficult to transfect particular cell types e.g., small cells, nonadherent cells |
| Physical | ● Direct injection ● Biolistic particle delivery ● Electroporation ● Laserfection | – Can be used for hard-to-transfect cells – May allow singlecell transfection – May allow precise insertion of nucleic acid into subcellular compartment | – May cause significant toxicity to cells – May require specialized equipment and operator skill |
| Biological | ● Virus-mediated | – High transfection efficiency – Can be used for difficult to transfect cells | – Requires biosafetystandard laboratory space – Risk of insertional mutagenesis – DNA insert size limit |
Factors affecting transfection efficiency
Several variables, including as cell health, confluence, and nucleic acid quality, might affect the efficacy of transfection.
- Cell health: Cells must be cultured in the needed medium at the proper circumstances, often at 37°C in an incubator with enough humidity and 5% CO2. On either the ATCC or DSMZ website, appropriate cell culture conditions for certain cell lines can be reviewed. Mycoplasma contamination of cells should be routinely screened for, as it not only affects transfection efficiency but can also lead to false results. Passage number may also influence the success of transfection; hence, a passage number 50 is often recommended, as cells may become less transfectable after multiple passages. Occasionally, it may be required to thaw and utilise new, uncontaminated stocks.
- Confluence of cells: The recommended ranges of cell confluence vary greatly (40–80%) depending on the cells utilised. Due to a lack of cell-to-cell interaction and/or insufficient paracrine impulses, too few cells will develop very slowly in general. Too many cells, in contrast, will result in contact inhibition and inadequate uptake of nucleic acid. In addition, actively dividing cells are better at absorbing nucleic acid than quiescent cells (although, as stated above, some transfection procedures may be appropriate for slowly growing and/or quiescent cells).
- Nucleic acid quality: Ensure that the nucleic acid utilised for transfection is devoid of contaminants, such as protein or microbiological components like as endotoxin. We typically prepare our DNA with Qiagen plasmid kits due of its high purity and low endotoxin contamination. If endotoxin is of special concern, DNA purification kits with low endotoxin levels are available (e.g., EndoFree plasmid kits, Qiagen).
- Number of Passages: Limit the number of paragraphs to below fifty. In addition, the number of cell passages employed in various tests should be uniform. With immortalised cell lines, cell properties might alter over time, and cells may not react to the same transfection conditions after multiple passages, resulting in low gene expression.
Optimization of transfection efficiency
There are a number of factors to consider when maximising your transfection efficiency, and depending on the experimental setup and/or desired outcome, this may be of greater or lesser importance. For instance, if low transfection efficiencies make it difficult to determine a biological effect, stable cell lines might be considered.
- Cell density: Try various cell densities to see which works best with a specific transfection reagent/method. When preparing cells for a transfection-based experiment, it is also crucial to consider the duration of the study. For instance, a typical siRNA knockdown experiment would be allowed for 72 hours before harvesting, and if you have fast-growing cells and would want them to be 70–80% confluent at the time of harvesting, you should change the initial cell density accordingly.
- Ratio of nucleic acid/transfection reagent: Each manufacturer of a given transfection reagent will propose a range of nucleic acid/transfection reagent ratios. When beginning transfections with a novel reagent, it is prudent to test multiple reagent:nucleic acid ratios (e.g., 1:1, 2:1, 3:1). Ideally, your chosen ratio should regularly provide you with high transfection effectiveness, minimum cell toxicity, and a cost-benefit balance.
- Cell culture settings: The culture conditions and growth characteristics of each cell type can affect the transfection efficiency. In addition, several cell types are notoriously difficult to transfect and necessitate the use of specific procedures and/or reagents (e.g., nonadherent cells). In addition, the interval between plating and transfection can affect the efficiency of uptake of nucleic acid/reagent complexes by adherent cells. We typically transfect our cells between 4 and 24 hours after plating, depending on the cell type and experimental requirements. We have discovered that significantly later time points can significantly reduce the efficacy of transfection.
- Measuring transfection efficiency: There are a variety of methods for determining the transfection efficiency. Direct imaging of a cell population, for example expressing pEGFP. In addition, a marker such as GFP expression can be utilised to sort cells on a FACS machine, allowing for the collection of the transfected population. In addition, cells can be transfected with constructs encoding for proteins such as -galactosidase or luciferase, enabling the use of colorimetric, fluorescent, chemiluminescent, or photon emission assays to measure transfection effectiveness. This information may be required if differences in transfection efficiency may affect the outcome of the experiment (e.g., if you are comparing multiple treatments, proteins, and/or time points).
What is Stable and transient transfection?
Stable and transient transfection have different long-term effects on a cell; a stably-transfected cell will continuously express transfected DNA and pass it on to daughter cells, whereas a transiently-transfected cell will express transfected DNA only briefly and will not pass it on to daughter cells.
For some uses of transfection, temporary expression of the transfected genetic material is adequate. Since transfection-introduced DNA is typically not incorporated into the nuclear genome, the foreign DNA will be diluted or destroyed during mitosis. Cell lines carrying the Epstein–Barr virus (EBV) nuclear antigen 1 (EBNA1) or the SV40 large-T antigen permit the episomal amplification of plasmids harbouring the viral EBV (293E) or SV40 (293T) origins of replication, therefore significantly decreasing the rate of dilution.
If the transfected gene is to remain in the genome of the cell and its daughter cells, a stable transfection must take place. To do this, a marker gene is co-transfected, which confers a selective advantage on the cell, such as tolerance to a specific toxin. By accident, some (very few) transfected cells will have incorporated foreign genetic material into their genome. Upon addition of the poison to the cell culture, only the few cells with the marker gene integrated into their genomes will be able to multiply, while the remaining cells would perish. After a period of time under this selective stress (selection pressure), only cells with stable transfection remain and can be further grown.
These are typical agents for selecting stable transfection:
- Geneticin, or G418, neutralized by the product of the neomycin resistance gene
- Puromycin
- Zeocin
- Hygromycin B
- Blasticidin S
RNA transfection
- RNA can also be transfected into cells to express its encoded protein transiently or to explore the kinetics of RNA degradation. RNA transfection is frequently utilised in non-dividing primary cells.
- siRNAs can also be transfected to inhibit RNA (i.e. loss of RNA and protein from the targeted gene). This has been a prominent use in research to “knock down” proteins of interest (such as Endothelin-1) with potential gene therapy applications. The toxicity of the transfection to cells and the potential “off-target” impact on the expression of other genes/proteins are limitations of the silencing strategy.
- RNA can be extracted from lysed cells or generated chemically or enzymatically using an RNA polymerase to transcribe a DNA template from free nucleotides. Similar to DNA, RNA can be administered to cells through microinjection, electroporation, or lipid-mediated transfection.
- If the RNA encodes a protein, transfected cells can convert the RNA into the encoded protein if the RNA encodes a protein. If the RNA is a regulatory RNA (such as a miRNA), it may cause additional cellular modifications (such as RNAi-mediated knockdown).
- Encapsulating the RNA molecule in lipid nanoparticles was a breakthrough in the production of effective RNA vaccines, overcoming a number of significant technical obstacles in the delivery of the RNA molecule into the human cell.
- RNA molecules shorter than around 25nt (nucleotides) elude detection by the innate immune system, which is activated by longer RNA molecules. The majority of cells in the body express proteins of the innate immune system, which, when exposed to exogenous long RNA molecules, generate inflammatory signalling cascades.
- This inflammation makes the exposed cell and neighbouring cells hypersensitive to subsequent exposure. Therefore, whereas a cell can be repeatedly transfected with short RNA with minimal non-specific consequences, repeatedly transfecting cells with even a modest amount of long RNA can result in cell death if the innate immune system is not suppressed or evaded (see “Long-RNA transfection” below).
- Biological research frequently employs short-RNA transfection to silence the expression of a target protein (using siRNA) or to express or inhibit the activity of a miRNA (using short RNA that operates independently of the cell’s RNAi machinery and is therefore not referred to as siRNA).
- Although DNA-based vectors (viruses, plasmids) that encode a short RNA molecule can also be utilised, short-RNA transfection does not pose a risk of altering the cell’s DNA; this has led to the development of short RNA as a new class of macromolecular pharmaceuticals.
- Long-RNA transfection is the intentional introduction of RNA molecules longer than approximately 25 nucleotides into live cells. Short-RNA transfection is distinguished from long-RNA transfection due to the fact that foreign long RNA molecules induce an innate immune response in cells, which can result in a number of nonspecific consequences such as translational inhibition, cell-cycle arrest, and death.
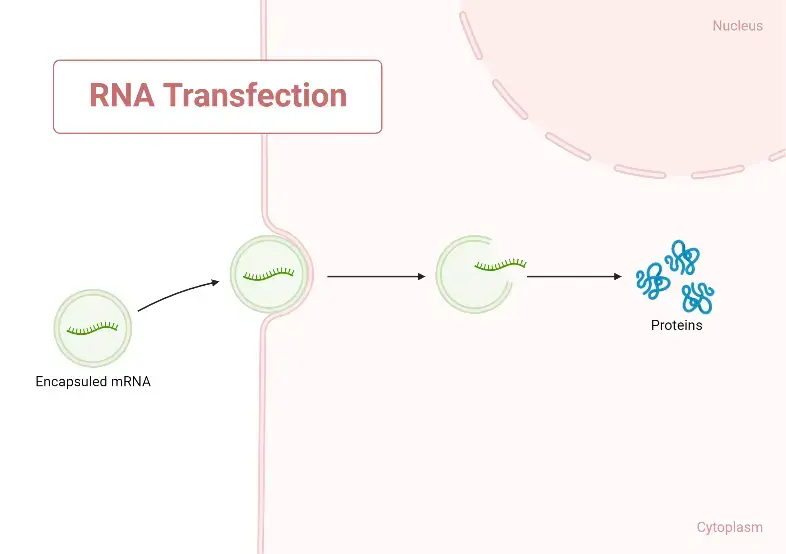
Transfection reagents
Transfection reagents are compounds that are used to introduce foreign DNA into cells for the purpose of genetic manipulation. There are several types of transfection reagents, including:
- Liposomes: Liposomes are spherical structures composed of a lipid bilayer that can complex with and deliver DNA into cells.
- Polyethylenimine (PEI): PEI is a positively charged polymer that can effectively neutralize the negative charge of DNA, allowing it to penetrate the cell membrane.
- Calcium phosphate: Calcium phosphate is a salt that can be used to precipitate DNA, which then complexes with cell membranes to allow uptake of the DNA.
- Electroporation: Electroporation involves applying an electric field to cells, temporarily permeabilizing the cell membrane and allowing the uptake of foreign DNA.
- Viral vectors: Virus-based systems, such as retroviral and lentiviral vectors, can be used to deliver foreign DNA into cells, although they may pose safety concerns due to the potential for viral replication.
The choice of transfection reagent depends on the type of cells being transfected, the desired outcome, and the availability of equipment and resources. Transfection reagents can be used for both permanent and transient transfection, depending on the intended application.
What is Repeated long-RNA transfection?
Interferon-, STAT2, and EIF2AK2 inhibition is sufficient to rescue human fibroblasts from cell death caused by repetitive transfection with lengthy, protein-encoding RNA. Interferon signalling inhibition impairs the positive feedback loop that typically makes cells hypersensitive to exogenous long RNA. Recently, this method was employed to express reprogramming proteins in primary human fibroblasts.
Application of Transfection
Transfection is the process of introducing foreign DNA into a cell, and it has a wide range of applications in cellular and molecular biology. Some of the main applications include:
- Gene expression analysis: Transfection is used to study the function of a specific gene by introducing it into a cell and observing changes in its expression.
- Protein production: Transfection can be used to produce large quantities of a specific protein for research or therapeutic purposes.
- Genetic engineering: Transfection is used to modify the genome of cells for various applications, such as developing cell lines for drug discovery, creating models of human disease, and creating new crops.
- Gene therapy: Transfection is used to introduce therapeutic genes into cells to treat genetic diseases.
- Vaccine development: Transfection is used to produce vaccines by introducing genes coding for viral antigens into cells, which can then be used to stimulate an immune response in patients.
Advantages of Transfection
Transfection is a process used to introduce genetic material into cells. The main advantages of transfection include:
- Studying gene function: Transfection allows researchers to study the function of specific genes by introducing them into cells and observing changes in cellular behavior.
- Creating cell lines: Transfection can be used to create stable cell lines that express a specific gene, which can be used for various applications such as drug discovery and vaccine development.
- Gene therapy: Transfection has the potential to be used in gene therapy to deliver therapeutic genes to treat genetic disorders or diseases.
- Study of diseases: Transfection allows researchers to study the underlying mechanisms of diseases by introducing disease-causing genes into cells and observing the effects.
- High-level protein expression: Transfection can be used to achieve high-level expression of proteins for research and industrial applications.
Overall, transfection provides an efficient and effective way to study gene function and has numerous applications in various fields of biotechnology and medicine.
Disadvantages of Transfection
Transfection is a useful tool for introducing genetic material into cells, but it also has some limitations and drawbacks, including:
- Low efficiency: Transfection efficiency can be low, with only a small percentage of cells actually taking up the introduced genetic material.
- Toxicity: Some transfection methods can cause toxicity to the cells, leading to reduced cell viability or altered cellular behavior.
- Variability: Transfection efficiency can vary greatly between cell types and can be affected by various factors such as the size and complexity of the genetic material being introduced.
- Limited types of cells: Some cell types are difficult or impossible to transfect, which limits the use of transfection in certain areas of research.
- Cost: Transfection can be a costly process, especially when using specialized reagents and equipment.
- Time-consuming: Transfection can be a time-consuming process, requiring multiple steps and sometimes multiple days to complete.
Overall, while transfection provides many benefits and opportunities for genetic research and biotechnology, it also has limitations and drawbacks that should be considered when choosing the appropriate method for a specific application.
Transit transfection
- Transfection is the process of introducing foreign DNA into cells for the purpose of genetic manipulation.
- Transit transfection refers to the transient expression of foreign DNA in the host cells, which means that the DNA is not integrated into the host genome and will only be present for a limited period of time.
- This method is useful for studying the function of genes or proteins without making permanent changes to the host genome.
Transient transfection
- Transient transfection is a method of introducing foreign DNA into cells for a limited period of time.
- The term “transient” refers to the fact that the DNA is not permanently integrated into the host genome, but instead will be present in the cell for a short period of time before being degraded or lost.
- This method is used for a variety of purposes, including the study of gene function, protein expression, and drug screening.
- Unlike permanent transfection, which involves integrating the foreign DNA into the host genome, transient transfection does not result in stable genetic modifications to the host cells.
What is pei for transfection?
PEI (Polyethylenimine) is a commonly used reagent for transient transfection. PEI is a polycationic polymer that can effectively complex and deliver foreign DNA into cells. The positive charge of PEI helps to neutralize the negative charge of DNA, allowing the DNA to penetrate the cell membrane and enter the cell. Once inside the cell, the DNA is expressed, allowing researchers to study gene function, protein expression, and other cellular processes. PEI has been widely used in various cell types, including primary cells and hard-to-transfect cell lines, and is considered to be one of the most efficient and widely used transfection reagents. However, as with any transfection method, the optimal conditions for PEI-mediated transfection, such as the PEI:DNA ratio, may vary depending on the cell type and the application.
What is plasmid transfection?
Plasmid transfection is the process of introducing a plasmid, a small circular piece of DNA, into a cell to temporarily alter its genetic material. This is often done for research purposes to study the expression of a particular gene, to over-express or knockdown a specific protein, or to create stable cell lines that continuously express the introduced gene.
Plasmids can be introduced into cells using a variety of methods, including physical methods such as electroporation or lipid-based methods such as lipofection. The success of plasmid transfection depends on several factors, including the type of cell, the size of the plasmid, and the transfection method used. The efficiency of plasmid transfection can be determined by measuring the expression of a reporter gene (such as green fluorescent protein) included on the plasmid, or by quantifying the amount of the desired protein produced.
How to determine transfection efficiency?
Transfection efficiency is the proportion of cells that successfully take up and express transfected genetic material (such as plasmids or siRNA). There are several methods to determine transfection efficiency, including:
- Fluorescent reporter genes: Expressing a fluorescent protein (e.g. GFP) as part of the transfected material and then measuring fluorescence intensity in individual cells to determine how many have successfully taken up the genetic material.
- Flow cytometry: Separating cells based on the expression of fluorescence and then analyzing the percentage of cells that express the fluorescent reporter gene.
- qPCR: Measuring the expression of a specific gene, such as a transfected gene or an endogenous control, and calculating the relative amount of the gene in transfected versus untransfected cells.
- Western blot: Analyzing the expression of a specific protein in transfected cells versus untransfected cells.
- Immunofluorescence: Staining cells with an antibody against the expressed protein to visualize transfection efficiency at the cellular level.
Transfection vs Transduction
| Feature | Transfection | Transduction |
|---|---|---|
| Definition | Introduction of exogenous DNA into eukaryotic cells | Introduction of exogenous DNA into bacterial or eukaryotic cells by a virus |
| Method | Physical (electroporation, lipofection) or chemical | Viral infection |
| DNA source | Plasmids, siRNA, mRNA | Viral genome |
| Integration | May or may not integrate into host genome | Usually integrates into host genome |
| Stable expression | Transient expression, stable expression possible with selection | Stable expression through integration |
| Specificity | Can be used for specific cells or tissues | Limited by host specificity of the virus |
Transfection refers to the process of introducing exogenous DNA into eukaryotic cells using physical or chemical methods, such as electroporation or lipofection. Transduction, on the other hand, refers to the introduction of exogenous DNA into cells by a virus. In transduction, the DNA is carried by the virus and is integrated into the host genome, leading to stable expression of the introduced gene. Transfection can be used to introduce specific genes into specific cells or tissues, while the specificity of transduction is limited by the host specificity of the virus.
Transfection vs Transformation
| Feature | Transfection | Transformation |
|---|---|---|
| Definition | Introduction of exogenous DNA into eukaryotic cells | Introduction of exogenous DNA into bacteria or plant cells |
| Method | Physical (electroporation, lipofection) or chemical | Chemical (competent cells) or physical (electroporation) |
| DNA source | Plasmids, siRNA, mRNA | Plasmids or genomic DNA |
| Integration | May or may not integrate into host genome | Integrates into host genome |
| Stable expression | Transient expression, stable expression possible with selection | Stable expression through integration |
| Specificity | Can be used for specific cells or tissues | Limited by cell type and DNA uptake efficiency |
Transfection refers to the process of introducing exogenous DNA into eukaryotic cells using physical or chemical methods, such as electroporation or lipofection. Transformation, on the other hand, refers to the introduction of exogenous DNA into bacteria or plant cells using chemical (such as competent cells) or physical methods (such as electroporation). The introduced DNA integrates into the host genome, leading to stable expression of the introduced gene. Transfection can be used to introduce specific genes into specific cells or tissues, while the specificity of transformation is limited by the cell type and DNA uptake efficiency.
Facts about Transfection
- Transfection is the process of introducing foreign DNA into eukaryotic cells, often with the aim of expressing a specific protein or studying gene function.
- The efficiency of transfection can vary greatly depending on the type of cell and the method used, with some methods achieving high levels of transfection efficiency, while others are less successful.
- The most commonly used methods of transfection include calcium phosphate precipitation, electroporation, and lipofection, each with its own advantages and disadvantages.
- Lipofection, which uses lipid-based delivery systems, is one of the most widely used transfection methods because of its high efficiency and ease of use.
- Another innovative method of transfection is viral transduction, which uses a viral vector to deliver foreign DNA into cells. This method is commonly used in gene therapy research.
- Transfection has a wide range of applications, including the production of therapeutic proteins, the study of gene function, and the development of new vaccines and treatments for diseases.
- Despite its numerous applications, transfection remains a challenging process, and there is ongoing research to develop more efficient and effective methods of delivery.
FAQ
What is Transfection?
Transfection is the process of introducing genetic material into a host cell. This is typically done with the goal of altering the expression of genes or studying their function. Transfection can be performed using various techniques, such as viral vectors, electroporation, and chemical methods.
What is Electroporation Transfection?
Electroporation transfection is a method of introducing DNA into cells using an electric field. The electric field causes a temporary permeabilization of the cell membrane, allowing the DNA to enter. Electroporation is a commonly used method in molecular biology, particularly in the study of gene function and regulation.
What is Chemical Transfection?
Chemical transfection is a method of introducing genetic material into cells using chemical reagents. These reagents help to permeabilize the cell membrane and allow the DNA to enter. Chemical transfection can be less efficient than other methods, such as electroporation, but it is less invasive and can be used on delicate cells that may be damaged by other transfection methods.
Why is Transfection Important?
Transfection is an important tool in molecular biology because it allows researchers to study gene function and regulation. By introducing new genetic material into cells, scientists can learn about the effects of specific genes, and use this information to develop new treatments for genetic diseases or to better understand cellular processes. In addition, transfection can be used to produce large quantities of proteins for use in research and biotechnology.
What are the Potential Risks of Transfection?
While transfection can be an effective tool for genetic research, it can also carry potential risks. One major concern is the potential toxicity of transfection reagents, which can damage or kill cells. In addition, introducing new genetic material into cells can alter their normal function, leading to unexpected consequences. It is important for researchers to carefully consider the risks of transfection and take steps to minimize them,
Why are hek293 cells used for transfection?
HEK 293 cells are widely used for transfection because of their high transfection efficiency and robust growth characteristics. The HEK 293 cell line was derived from human embryonic kidney cells and has been engineered to express the adenoviral E1A and E1B genes, making it highly permissive for the introduction of foreign DNA. This results in a high rate of successful transfection compared to other cell lines, making it a commonly used cell line for the study of gene expression, protein production, and other cellular processes. Additionally, HEK 293 cells grow quickly and are easy to maintain in the laboratory, making them a convenient choice for many researchers. Overall, the combination of high transfection efficiency, fast growth rate, and ease of maintenance make HEK 293 cells a popular choice for transfection experiments.
What is transfection efficiency?
Transfection efficiency refers to the proportion of cells that have successfully taken up and expressed foreign DNA following a transfection procedure. The efficiency is typically expressed as a percentage of the total number of cells in the sample that have been successfully transfected. A high transfection efficiency means that a large proportion of cells have taken up and expressed the foreign DNA, while a low transfection efficiency means that only a small proportion of cells have been successfully transfected. The transfection efficiency is an important factor to consider in transfection experiments, as it can impact the results of the study and the interpretation of the data. The transfection efficiency can be influenced by many factors, including the type of transfection reagent, the size and type of the foreign DNA, the cell type, and the conditions of the transfection procedure.
References
- Sheikh, S., Coutts, A. S., & La Thangue, N. B. (2017). Transfection. Basic Science Methods for Clinical Researchers, 191–209. doi:10.1016/b978-0-12-803077-6.00011-4
- Chong ZX, Yeap SK, Ho WY. Transfection types, methods and strategies: a technical review. PeerJ. 2021 Apr 21;9:e11165. doi: 10.7717/peerj.11165. PMID: 33976969; PMCID: PMC8067914.
- https://www.thermofisher.com/in/en/home/references/gibco-cell-culture-basics/transfection-basics/introduction-to-transfection.html
- https://www.promega.in/resources/guides/cell-biology/transfection/
- https://www.polyplus-transfection.com/about-us/what-is-transfection/
- https://www.vedantu.com/biology/transfection
- https://www.sigmaaldrich.com/IN/en/technical-documents/technical-article/cell-culture-and-cell-culture-analysis/transfection-and-gene-editing/transfection-reagents
- https://altogen.com/transfection-resource/
- https://bitesizebio.com/9288/principles-and-mechanisms-of-mammalian-cell-transfection/
- https://www.bio-rad.com/webroot/web/pdf/lsr/literature/10-0826_transfection_tutorial_interactive.pdf
- https://ibidi.com/content/263-chemical-transfection
- https://www.genscript.com/what-is-dna-transfection.html
- https://www.frontiersin.org/articles/10.3389/fbioe.2021.701031/full