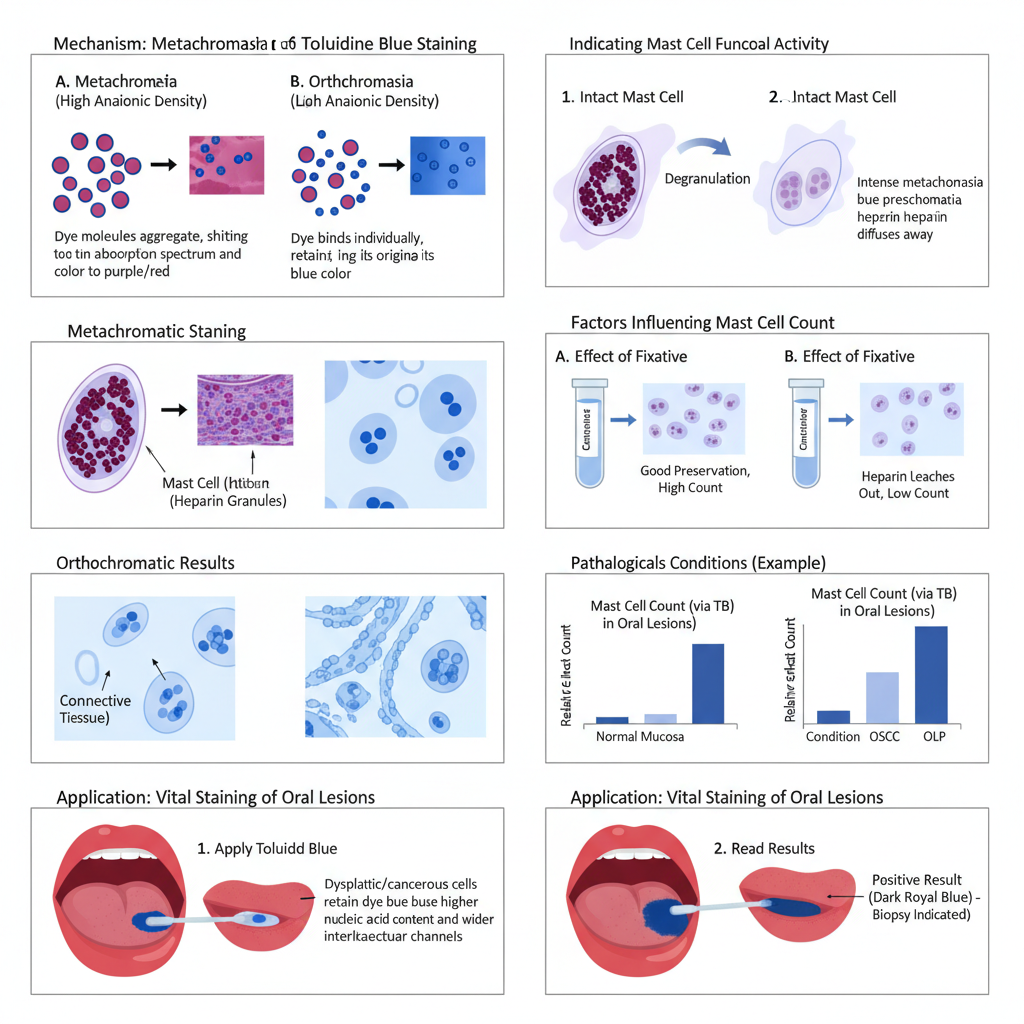
Toluidine blue staining is a special histological staining method in which a basic thiazine dye is used to identify different acidic components present in the tissue. It is the process where the dye binds with negatively charged groups and most of the nucleic acids stain blue as these are orthochromatic in nature. It is the metachromatic property of toluidine blue that makes the staining useful, because in some tissues the dye molecules is aggregated on the dense anionic sites and the colour is changed into dark purple or reddish-violet.
This is referred to as metachromasia. In mast cells this process occur when the dye interacts with the sulfated glycosaminoglycan heparin present in the granules, and the granules is stained in purple colour.
It is carried out in very low pH conditions (pH≤2) so that only the highly sulfated groups of mast cell granules is stained and the background staining is reduced. It is important that proper fixatives are used because routine aldehyde fixatives can cause the loss of heparin and the mast cell number is underestimated.
Intact mast cells show strong metachromatic staining but after degranulation the released granules lose the charge density and appear bluish or pink in orthochromatic form. It is a rapid and inexpensive staining method used to detect mast cells, mucins and cartilage, and it is also used as a vital stain for identifying dysplastic or cancerous lesions in vivo.
Principle of Toluidine blue stain
The principle of Toluidine blue stain is based on a property called metachromasia, and it is the process where the basic dye changes its original blue colour when it binds with certain tissue components. Toluidine blue is a cationic thiazine dye, so it normally binds with acidic groups and stains them in blue, which is referred to as orthochromatic staining. It is the metachromatic reaction that makes the stain important, because some components such as mast cell granules contain strongly acidic sulfated groups that attract a large number of dye molecules. In this condition the dye molecules is aggregated on the dense negative charges and the absorption spectrum is shifted. This is the process by which the colour is changed into dark purple or reddish-violet. The major chromotrope in mast cells is the sulfated glycosaminoglycan heparin, and the high charge density present in heparin helps in the aggregation of the dye molecules.
It is carried out in very low pH conditions (pH≤2) so that the weaker acidic groups present in other tissues become protonated and cannot bind the dye. Only the highly sulfated groups remain ionized and these produce the metachromatic colour.
When the mast cells undergo degranulation, the heparin diffuses and the charge density is lost, so the dye now stains in blue or pink form which is orthochromatic. It is important that the tissue is fixed properly because water-soluble glycosaminoglycans can be lost during aldehyde fixation and the mast cells is underestimated. Thus the principle of the stain depends on the dye-to-dye aggregation on strong anionic sites, forming the characteristic metachromatic reaction.
Requirement
- Toluidine blue dye (basic thiazine type used for metachromatic staining).
- Aqueous toluidine blue solutions prepared in different concentrations (0.1%, 0.5% or 1%).
- Acidic toluidine blue solution used for metachromasia, generally prepared in low pH conditions.
- Hydrochloric acid (HCl) and glacial acetic acid used for preparing acidic dye solutions.
- Carnoy’s fluid used as a fixative for preserving mast cell granules.
- Mota’s basic lead acetate fixative for preventing the loss of sulfated glycosaminoglycans.
- Formalin-based fixatives used in routine work but these are not suitable for proper quantification.
- Ethyl alcohol solutions (70%, 95% and absolute alcohol) used during dehydration steps.
- Distilled water used for preparing different solutions.
- Clearing agents such as xylene used before mounting.
- Mounting medium like DPX used for coverslipping because aqueous mediums can remove the metachromatic colour.
- Control slides containing mast cells used to check the staining quality.
Procedure of Toluidine Blue Staining
Toluidine Blue staining is used for demonstrating mast cell granules and other tissue components showing metachromasia. It is applied on paraffin sections as well as on frozen sections. It is the process where the dye binds with highly sulfated groups and gives a characteristic metachromatic colour.
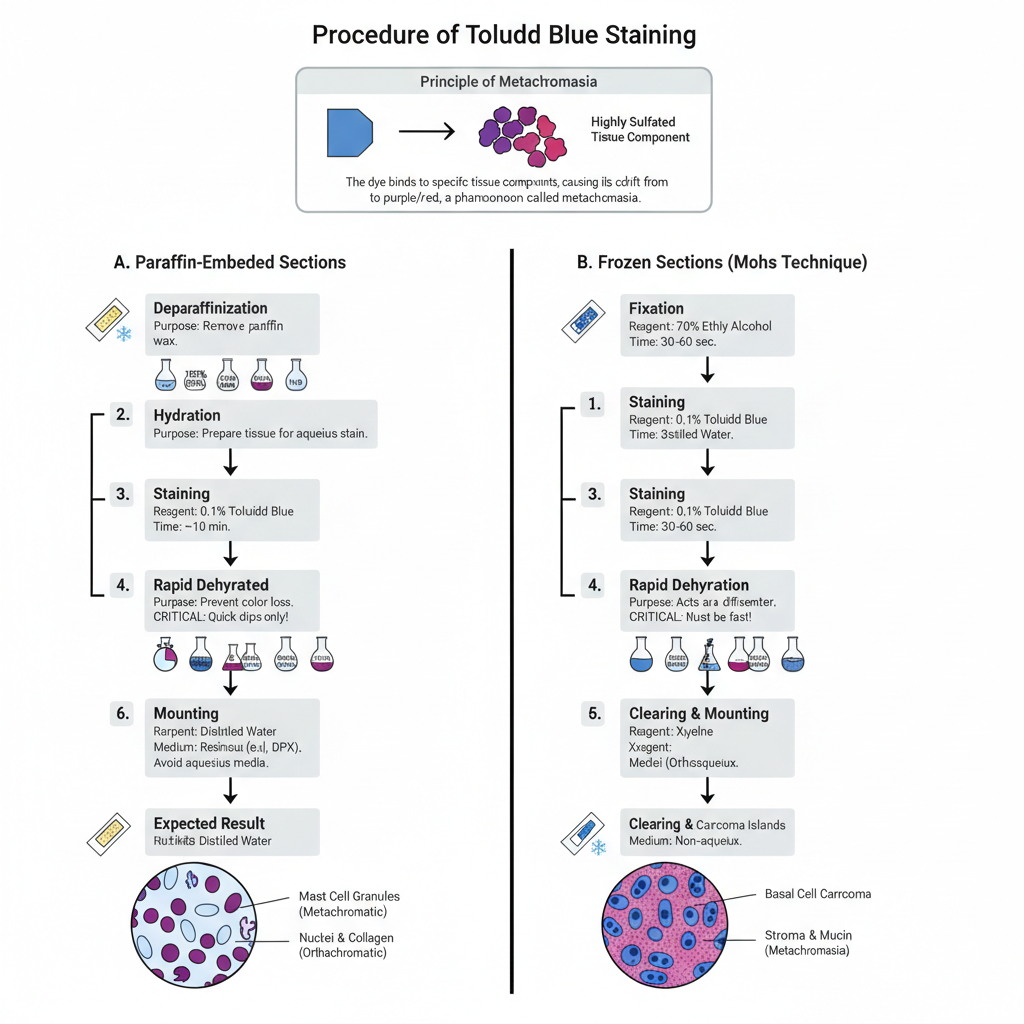
A. Staining of Paraffin-Embedded Sections
Steps
- Deparaffinization– The paraffin from the tissue section is removed by placing the slides in xylene. Usually, 3 changes are used and each change is kept for few minutes. It is important that the wax is completely removed.
- Hydration– The sections is passed through descending grades of alcohol (100% to 95%) and then transferred to distilled water. It is the step that prepares the tissue for staining.
- Washing– The slides are washed well in distilled water.
- Staining– The sections are placed in Toluidine Blue solution.- Some of the main conditions are–
- For routine staining, 0.1% aqueous Toluidine Blue is used for about 10 minutes.
- For strong metachromasia, acidified Toluidine Blue solution (0.5% in 0.5M HCl, pH ≤2.0) is used and the staining time is longer.
- Rinsing– The stained slides are washed in distilled water immediately.
- Rapid Dehydration– In this step, the slides are quickly passed through 95% alcohol and then absolute alcohol. This step is crucial as any delay causes the metachromatic colour to leach out. Usually 1 second dip is maintained.
- Clearing– The sections are placed in xylene for clearing. Around 3 changes are used.
- Mounting– The stained tissue is mounted with a resinous mounting medium (DPX). Aqueous mounting medium is avoided as it results in loss of metachromatic colour.
Expected Result
- Mast cell granules appear deep violet or reddish-purple (metachromatic).
- Nuclei and collagen fibres appear blue (orthochromatic).
B. Staining of Frozen Sections (Mohs Technique)
Steps
- Fixation– Frozen sections (5–7 µm) are fixed in 70% ethyl alcohol for about 30–60 seconds.
- Washing– The slides are washed in distilled water.
- Staining– The sections are stained in 0.1% Toluidine Blue (aqueous). It is kept for 30–60 seconds depending on intensity required.
- Washing– A gentle wash is given in distilled water.
- Rapid Dehydration– A quick dip in 95% alcohol followed by two changes of absolute alcohol is done. Alcohol acts as differentiator so the step must be fast.
- Clearing and Mounting– The sections are cleared in xylene and mounted with non-aqueous medium.
Expected Result
- Basal cell carcinoma islands show deep blue to purple colour.
- Stroma and mucopolysaccharides appear pink to magenta (metachromasia).
- Nuclei appear dark blue.
Results of Toluidine Blue Staining
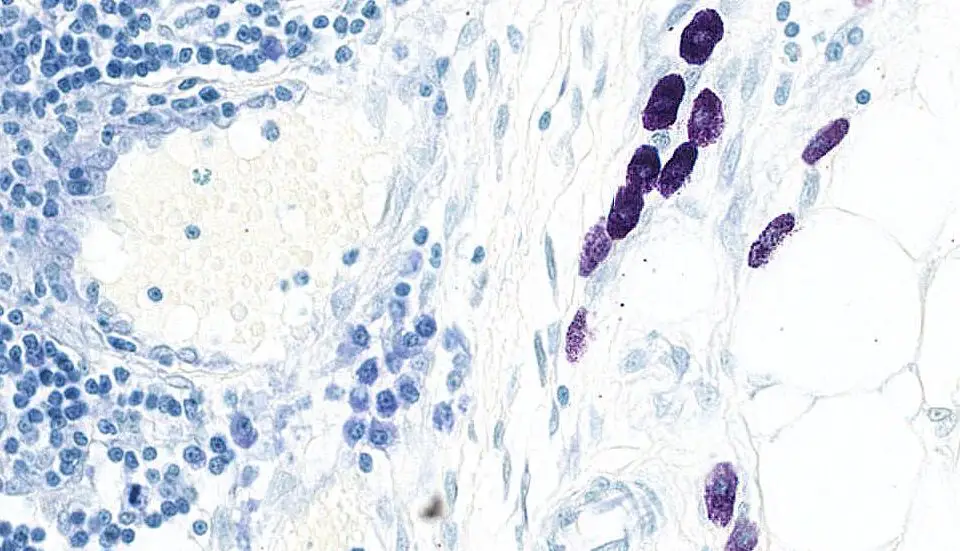
Toluidine Blue staining gives two characteristic colour reactions because the dye shows metachromasia. It is the process where highly anionic groups in tissues make the dye molecules aggregate and change their colour. The tissue components therefore appear in metachromatic or orthochromatic shades depending on their chemical nature.
A. Metachromatic Results
Metachromasia is the diagnostic feature. It is observed when the dye binds with structures having densely packed negatively charged groups.
Some of the main structures showing metachromasia are–
- Mast cell granules appear dark purple, reddish-purple or red-violet. It is due to the presence of highly sulfated heparin inside the granules.
- The mast cells appear as large mononuclear cells. They may be oval, elongated or fusiform and the nucleus sometimes becomes indistinct because of the dense granules.
- Acid mucins and connective tissue mucins show purple to red shades.
- Cartilage matrix stains purple because of its glycosaminoglycan content.
- Endocrine cell granules and sulfatides may show purple reactions.
This metachromatic reaction is lost if the granules discharge their contents.
B. Orthochromatic Results
Orthochromasia means the dye retains its original blue colour. It is seen when the tissue does not have sufficient anionic density.
These are–
- Background connective tissue appears blue.
- Nuclei stain sky-blue or dark blue depending on the section.
- Corynebacterium diphtheriae granules can show red-violet.
- Helicobacter stains dark blue.
C. Results Related to Mast Cell Activity
Toluidine Blue staining also indicates the functional condition of mast cells.
- Intact mast cells show an intense dark violet or red-violet colour because the heparin content inside the granules remains preserved.
- Degranulated mast cells lose metachromasia. When granules discharge, the heparin diffuses and the colour changes to pale pink or diffuse blue-violet.
- In different oral lesions such as OLP, OSCC, IFH and OPG, the number of degranulated mast cells has been found higher than intact cells.
D. Results in Different Pathological Conditions
Toluidine Blue is used for mast cell count in many lesions. But the count is generally lower compared to immunohistochemical marker MCT because TB cannot show degranulated mast cells.
Some results are–
- In Pyogenic Granuloma, the mean mast cell count with TB is lower compared to MCT.
- In Gingival Hyperplasia and Pericoronitis, similar lower counts are observed with TB whereas MCT shows higher values.
- Among PG, GH and Pericoronitis, TB does not show significant difference, but MCT does.
- In Oral Squamous Cell Carcinoma, the TB count becomes significantly high compared to normal mucosa.
- In some studies, OLP showed the highest mean mast cell count among OLP, OSCC, IFH and OPG.
E. Results Depending on Fixation
The number of mast cells detected depends greatly on the fixative.
- Carnoy’s fixative preserves metachromasia very well and shows a high number of mast cells.
- Formalin-fixed tissues show a very low count because heparin can leach out.
- In canine and feline tissues, better mast cell detection is observed in Carnoy’s and Mota’s basic lead acetate compared to formalin.
F. Results of Vital Staining
Toluidine Blue is used as a vital stain in the oral cavity for detecting dysplasia and carcinoma.
Some of the main findings are–
- Positive result is shown by a dark royal blue or deep blue area that remains after rinsing.
- Light blue staining is considered doubtful.
- Dysplastic and neoplastic cells retain the stain more because they have increased nucleic acids or wider intracellular channels.
- It helps in marking suspicious oral lesions and selecting biopsy sites.
- Sensitivity is high while specificity becomes low because inflammatory lesions can retain the dye falsely.
Uses of Toluidine Blue Staining
- It is used to detect mast cells in different tissues because the granules show strong metachromasia (purple or red-violet).
- It is used for mast cell count in oral lesions, reproductive organs and inflammatory tissues where intact and degranulated mast cells can be differentiated.
- It is applied to study mast cell activity in conditions like Oral Lichen Planus, OSCC, Inflammatory Fibrous Hyperplasia, Pyogenic Granuloma and Pericoronitis.
- It is used in veterinary pathology for identifying mast cells in canine and feline uterus and ovary, especially when tissues are fixed in Carnoy’s or Mota’s fixative.
- It is used to stain acidic mucins and other acid mucopolysaccharides which appear purple to red.
- It helps in staining cartilage matrix because of the high content of glycosaminoglycans.
- It stains endocrine cell granules and sulfatides showing purple to red-brown colour.
- It can demonstrate microorganisms, such as staining Corynebacterium diphtheriae granules (red-violet) and Helicobacter (dark blue).
- It is used for rapid staining of frozen sections, especially in Mohs surgery where basal cell carcinoma islands appear deep blue to purple.
- It is used as a vital stain in the oral cavity for identifying dysplasia and carcinoma by showing dark blue retention in malignant epithelium.
- It helps in selecting biopsy sites and marking the margin or extent of suspicious lesions during oral examination.
- It supports follow-up in oral cancer patients by detecting recurrent or second primary lesions.
- It is used in diagnosing mastocytosis, where atypical mast cells are easily recognized due to their metachromatic granules.
- It is used to study polyanionic components, such as chromatin alterations and extracellular proteoglycan complexes.
- It is used in antimicrobial photodynamic therapy (aPDT) as Toluidine Blue O works as a photosensitizer and helps in destroying resistant microorganisms like Candida and Staphylococcus.
Advantages of Toluidine Blue Staining
- It is a low-cost staining method and the dye is easily available in most laboratories.
- It is simple to perform and the staining procedure takes very little time, especially in frozen sections.
- It gives rapid results, making it suitable for quick evaluation in Mohs surgery and other immediate examinations.
- It clearly identifies mast cells, as the heparin-rich granules show strong metachromasia.
- It provides a good contrast, where mast cells appear purple or red-violet against a blue background.
- It helps in observing mast cell granules due to the clear and dense metachromatic appearance.
- It shows the functional status of mast cells, since intact cells stain metachromatically and degranulated cells stain orthochromatically.
- It acts as a useful histochemical stain for demonstrating mucins, cartilage matrix and other acidic components.
- It helps in studying changes in chromatin and extracellular proteoglycan complexes during various physiological events.
- It is used as a vital stain in the oral cavity, helping to detect dysplasia and carcinoma in living tissues.
- It assists in outlining lesions, showing the extent of abnormal epithelium before surgical excision.
- It helps in selecting biopsy sites by marking the areas that retain deep blue staining.
- It supports cancer surveillance, especially in high-risk individuals for oral malignancy.
- It is better than H&E stain for identifying mast cells, which are otherwise difficult to observe.
- It gives superior contrast compared to many other histochemical stains, showing higher mast cell density in different lesions.
Limitations of Toluidine Blue Staining
- It is highly sensitive to fixation, and the dye loses metachromasia if formalin is used, causing heparin to leach out from mast cell granules.
- It leads to underestimation of mast cell number, because the water-soluble granule contents diffuse during routine processing.
- It depends greatly on non-aldehyde fixatives, and proper mast cell staining is seen mainly with Carnoy’s fluid or Mota’s basic lead acetate.
- It shows much lower mast cell counts than IHC, since immunohistochemical markers like mast cell tryptase detect both intact and degranulated cells.
- It is not specific for mast cells, because basophils and some macrophages can also show metachromatic staining.
- It stains many other acidic structures, such as goblet cells, mucins and other GAG-rich components, reducing selectivity.
- It is technique-dependent, as prolonged staining can cause loss of selectivity and lead to metachromasia in other cell types such as eosinophils.
- It fails to detect degranulated mast cells, because the expelled granules lose the high anionic density needed for metachromasia.
- It cannot identify immature mast cells easily, since they contain less heparin and require long staining periods for proper visualization.
- It may provide inferior contrast, and other stains such as MGG or Astra blue can show mast cells more clearly in some tissues.
- It can give false-positive results in vital staining, because inflammatory or ulcerated tissues may retain the dye.
- It may miss early premalignant changes, as some studies reported high false-negative values when detecting early lesions.
- Buckley, M., & Walls, A. F. (2008). Identification of mast cells and mast cell subpopulations. Methods in Molecular Medicine, 138, 285–297. https://doi.org/10.1007/978-1-59745-366-0_24
- D’Ilario, L., & Martinelli, A. (2006). Toluidine blue: Aggregation properties and structural aspects. Modelling and Simulation in Materials Science and Engineering, 14(4), 581–595. https://doi.org/10.1088/0965-0393/14/4/003
- Fong, M., & Crane, J. S. (2025). Histology, mast cells. In StatPearls [Internet]. StatPearls Publishing.
- Gaur, S., & Sinha, O. N. (2015). Histological and histochemical observations on mast cell and glands in chronic tonsillitis. [Journal title not specified in excerpt], 71(Suppl 1), 32–37. https://doi.org/10.1007/s12070-015-0961-1
- Grigorev, I. P., & Korzhevskii, D. E. (2021). Modern imaging technologies of mast cells for biology and medicine (review). Sovremennye Tehnologii v Medicine/Modern Technologies in Medicine, 13(4), 93–107. https://doi.org/10.17691/stm2021.13.4.10
- Hamouzova, P., Cizek, P., Bartoskova, A., & Novotny, R. (2020). Different fixative solutions in the detection of mast cells in the canine and feline reproductive organs. Folia Morphologica, 79(2), 265–271. https://doi.org/10.5603/fm.a2019.0097
- Jain, A., Jaiswal, S., Vikey, A., Bagulkar, B., Bhat, A., & Shujalpurkar, A. (2022). Quantification of mast cells in nonreactive and reactive lesions of gingiva: A comparative study using toluidine blue and immunohistochemical marker mast cell tryptase. Journal of Oral and Maxillofacial Pathology, 25(3), 550–551. https://doi.org/10.4103/jomfp.JOMFP_267_19
- Mutsaddi, S., Kotrashetti, V. S., Nayak, R. S., & Pattanshetty, S. M. (2019). Comparison of histochemical staining techniques for detecting mast cells in oral lesions. Biotechnic and Histochemistry, 94(6), 459–468. https://doi.org/10.1080/10520295.2019.1597986
- Natesan, S. C., George, J., Krishnapillai, R., Ramakrishnan, B. P., & Thomas, P. (2017). Mast cell density in oral lesions using metachromatic stains: A comparative study. Journal of Clinical and Diagnostic Research, 11(10), ZC17–ZC19. https://doi.org/10.7860/JCDR/2017/30460.10744
- Newcomer Supply. (2025). Toluidine Blue Stain for Mast Cells – Technical Memo (Part 14027). Author.
- Newcomer Supply. (2025). Toluidine Blue Stain for Mohs Technique – Technical Memo (Part 14027 Revised April 2025). Author.
- Padra, J. T., & Lindén, S. K. (2021). Optimization of Alcian blue pH 1.0 histo-staining protocols to match mass spectrometric quantification of sulfomucins and circumvent false positive results due to sialomucins. Glycobiology, 32(1), 6–10. https://doi.org/10.1093/glycob/cwab091
- Pardanani, A. (2013). How I treat patients with indolent and smoldering mastocytosis (rare conditions but difficult to manage). Blood, 121(16), 3085–3094. https://doi.org/10.1182/blood-2013-01-453183
- Sridharan, G., & Shankar, A. A. (2012). Toluidine blue: A review of its chemistry and clinical utility. Journal of Oral and Maxillofacial Pathology, 16(2), 251–255. https://doi.org/10.4103/0973-029X.99081
- Strobel, S., Miller, H. R., & Ferguson, A. (1981). Human intestinal mucosal mast cells: evaluation of fixation and staining techniques. Journal of Clinical Pathology, 34(8), 851–858. https://doi.org/10.1136/jcp.34.8.851
- Zanotti, R., & Tanasi, I. (2021). Systemic mastocytosis: Multidisciplinary approach. Mediterranean Journal of Hematology and Infectious Diseases, 13(1), e2021068. https://doi.org/10.4084/MJHID.2021.068
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.