What is Telophase?
Telophase, derived from the Ancient Greek terms τέλος (télos) meaning ‘end’ and φάσις (phásis) signifying ‘appearance’, represents the concluding phase in the intricate process of cell division, both in mitosis and meiosis of eukaryotic cells. This phase is characterized by a series of events that essentially reverse the changes observed during prophase and prometaphase.
Key Features of Telophase:
- Nuclear Envelope Reformation: As telophase commences, the separated chromosomes or chromatids, having reached their respective poles, are enclosed by a reassembling nuclear envelope. The exact mechanism underlying this reformation remains a subject of scientific inquiry. Some theories postulate that vesicles of the disintegrated nuclear membrane receive signals to coalesce, akin to monomers forming a polymer. Alternatively, it is hypothesized that the endoplasmic reticulum, containing remnants of the old nuclear membrane, wraps around the clustered chromosomes, leading to the reformation of the nuclear envelope.
- Chromosomal Decondensation: Within the newly formed nuclear envelope, the tightly packed chromosomes commence relaxation, transitioning back to a more diffuse chromatin state, which is characteristic of the interphase period.
- Nucleolar Regeneration: The nucleolus, a specialized structure primarily involved in ribosome synthesis, re-emerges. Comprising a dense amalgamation of enzymes, RNA, and DNA, the nucleolus plays a pivotal role in ribosome formation – the cellular machinery responsible for protein synthesis.
- Microtubule Depolymerization: A salient feature of telophase is the disintegration of specific spindle microtubules. These microtubules, primarily composed of α-tubulin and β-tubulin subunits, disassemble upon receiving cellular signals. While some microtubules disassociate, others integral to cytokinesis remain intact.
- Duration: Telophase constitutes a relatively brief segment of the cell cycle, accounting for approximately 2% of its total duration.
Significance and Subsequent Processes:
Telophase serves as a precursor to cytokinesis, a process that typically initiates before the culmination of telophase. Cytokinesis ensures the segregation of the two nascent nuclei into distinct daughter cells, thereby completing cell division.
In the context of meiosis, telophase manifests in two distinct stages: telophase I and telophase II. These stages facilitate the separation of duplicate genetic material housed within the parent cell’s nucleus, culminating in the formation of two genetically identical daughter cells.
Molecular Drivers: The progression of telophase is primarily orchestrated by the dephosphorylation of substrates associated with mitotic cyclin-dependent kinase (Cdk).
In summation, telophase is a critical phase in cell division, ensuring the accurate segregation of genetic material and the restoration of cellular structures, setting the stage for the birth of new cells.
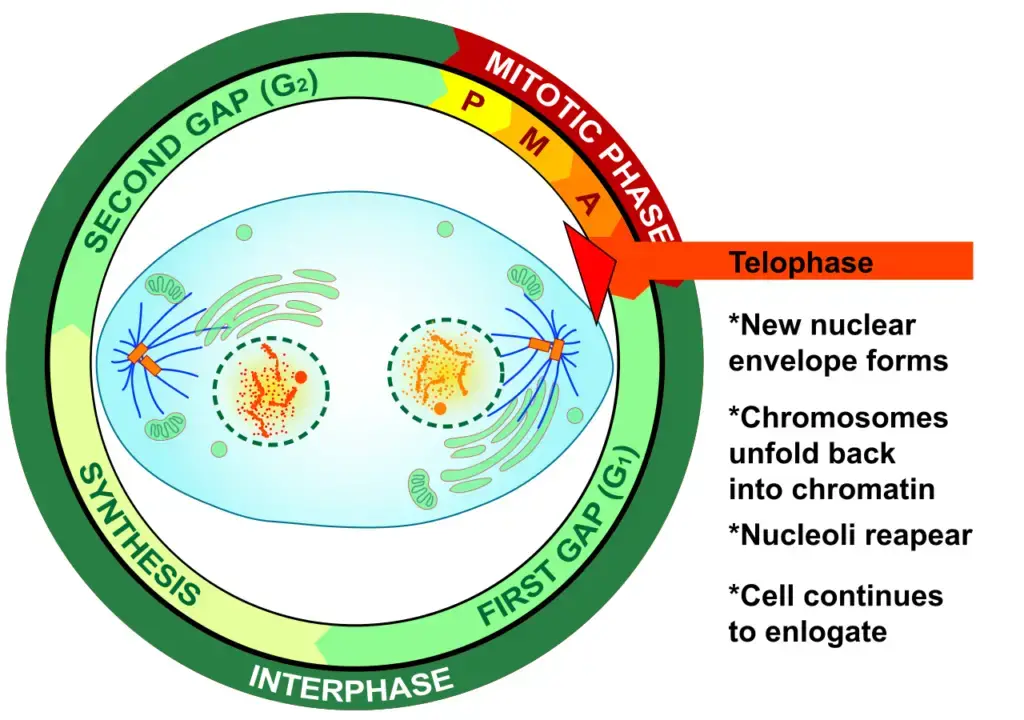
Definition of Telophase
Telophase is the penultimate stage in cell division, during which chromosomes decondense, spindle fibers disintegrate, and new nuclear envelopes form around the separated genetic material, preparing the cell for final division through cytokinesis.
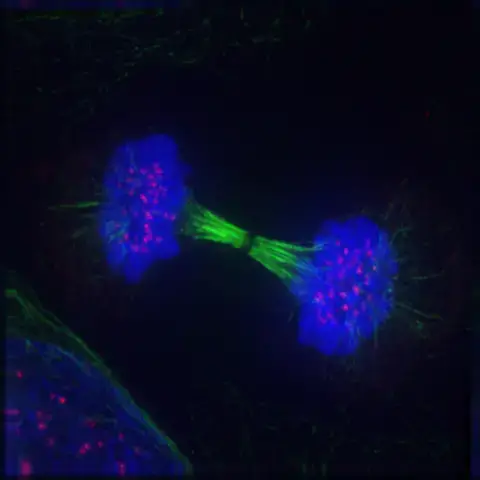
What happens during Telophase?
During telophase, a series of intricate cellular events transpire. Firstly, a nuclear envelope begins to assemble around each cluster of chromosomes, effectively segregating the nuclear genetic material from the surrounding cytoplasm. Concurrently, the previously condensed chromosomes initiate a process of decondensation, transitioning into a more diffuse and relaxed chromatin state. Following these events, telophase seamlessly transitions into cytokinesis, a phase responsible for partitioning the parent cell’s cytoplasm, culminating in the formation of two distinct daughter cells.
Timing of mitotic and meiotic telophases
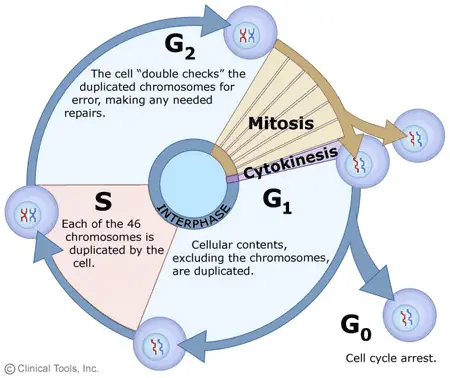
In the intricate process of cell division, the timing and sequence of events are paramount.
Mitotic Telophase: In mitosis, telophase serves as a transitional phase, situated between anaphase and cytokinesis. Following the separation of chromosomes during anaphase, telophase initiates, characterized by nuclear envelope reformation and chromosomal decondensation. Subsequent to telophase, cytokinesis ensues, wherein the cell’s cytoplasm undergoes division, resulting in the formation of two genetically identical daughter cells.
Meiotic Telophase: Meiosis, a more complex form of cell division, comprises two distinct stages: Meiosis I and Meiosis II.
- Telophase I: This phase follows anaphase I. Upon the conclusion of telophase I, a brief cytokinesis occurs, leading directly into the commencement of prophase II. This sequence ensures the cell is primed for the second meiotic division.
- Telophase II: Positioned within the framework of the second meiotic division, telophase II is preceded by anaphase II. Post telophase II, cytokinesis takes place, culminating in the formation of four non-identical daughter cells, each harboring half the genetic content of the original parent cell.
In summary, the timing of telophases, both mitotic and meiotic, is meticulously orchestrated, ensuring the accurate and efficient segregation of genetic material and the eventual formation of daughter cells.
Telophase in Mitosis
Mitosis, a fundamental process in the life cycle of eukaryotic cells, ensures the accurate replication and distribution of genetic material. Telophase, the concluding phase of mitosis, is instrumental in the culmination of this process, marking the final preparatory steps before the physical division of the cell.
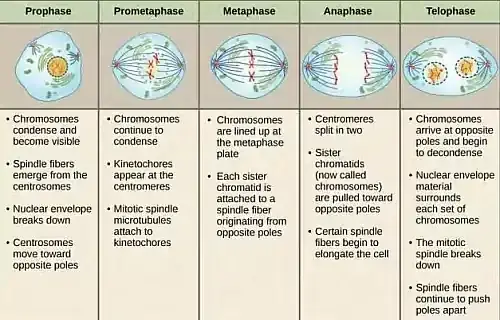
Key Events of Telophase in Mitosis:
- Chromosome Decondensation: After the rigorous activities of earlier mitotic stages, chromosomes, which were previously in a highly condensed state, begin to relax during telophase. This decondensation transforms them back into a more diffuse chromatin structure, facilitating gene expression and cellular functions in the subsequent interphase.
- Reformation of the Nuclear Envelope: A hallmark of telophase is the re-emergence of the nuclear envelope. This envelope, which had disintegrated in the initial stages of mitosis, reassembles around the separated chromosome sets. This reformation serves dual purposes:
- It provides a protective barrier for the genetic material, shielding it from potential cytoplasmic interferences.
- It reinstates the nuclear compartmentalization, ensuring that specific nuclear processes can resume in a controlled environment. The early stages of this reformation are characterized by the association of nuclear pore scaffold proteins with chromatin.
- Emergence of the Nucleolus: The nucleolus, vital for ribosome synthesis, reappears within the reforming nucleus. This resurgence ensures the efficient production of ribosomes, which are paramount for protein synthesis in the daughter cells.
- Mitotic Spindle Breakdown: The mitotic spindle, having played its role in chromosome separation, undergoes disassembly. This not only allows the chromosomes to spread within the nascent nuclei but also reallocates cellular resources for other vital functions.
- Onset of Cytokinesis: While not an intrinsic part of telophase, cytokinesis often commences in tandem with this phase. This process involves the division of the cell’s cytoplasm, facilitated by a contractile ring comprising actin and myosin. The ring’s contraction results in the inward pinching of the cell membrane, forming a cleavage furrow. As this furrow deepens, it eventually divides the cell into two distinct daughter cells, each enveloped by its own cell membrane.
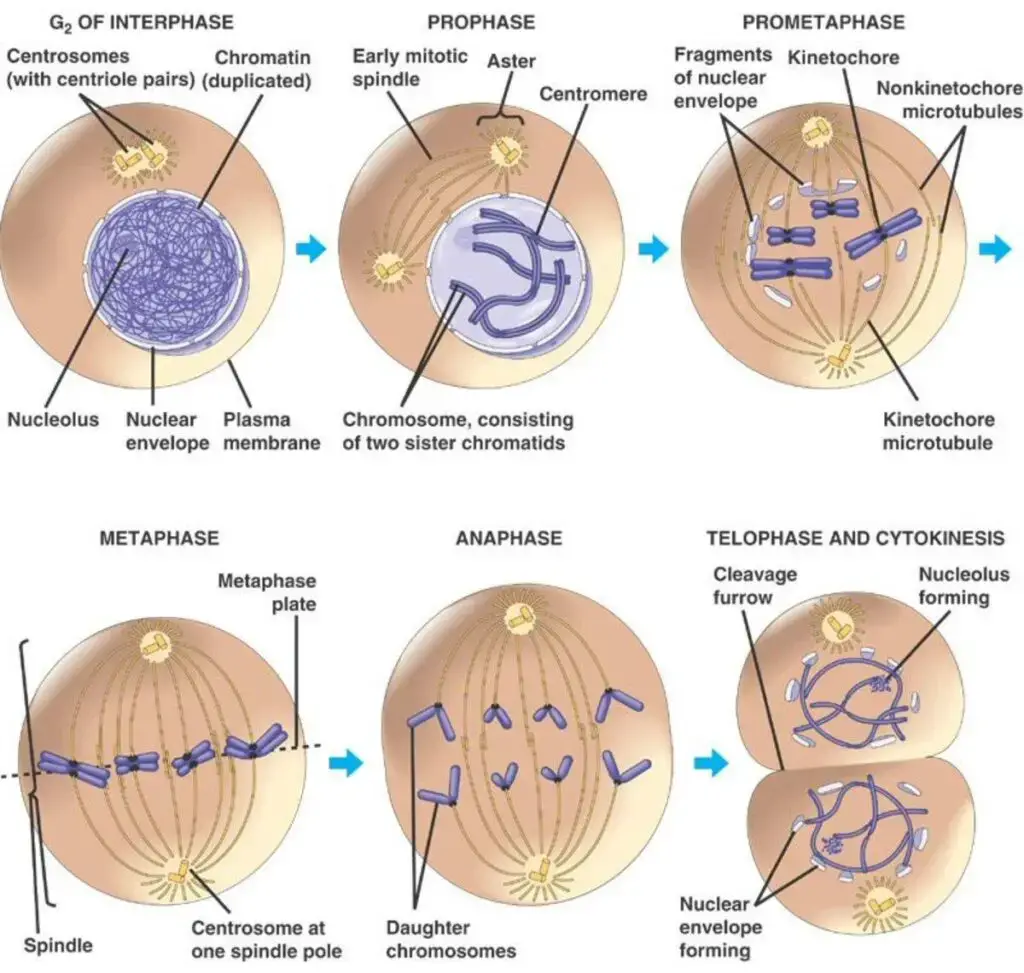
In summation, telophase in mitosis is a meticulously coordinated phase, ensuring the proper segregation of genetic material and the restoration of cellular structures, setting the stage for the birth of two genetically identical daughter cells.
Telophase In Meiosis
Meiosis, a specialized form of cell division, is instrumental in producing gametes for sexual reproduction. Unlike mitosis, which ensures growth and repair, meiosis aims to generate genetic diversity and halve the chromosome number. The telophase stage in meiosis, occurring twice as telophase I and telophase II, is distinct in its objectives and outcomes when compared to mitotic telophase.
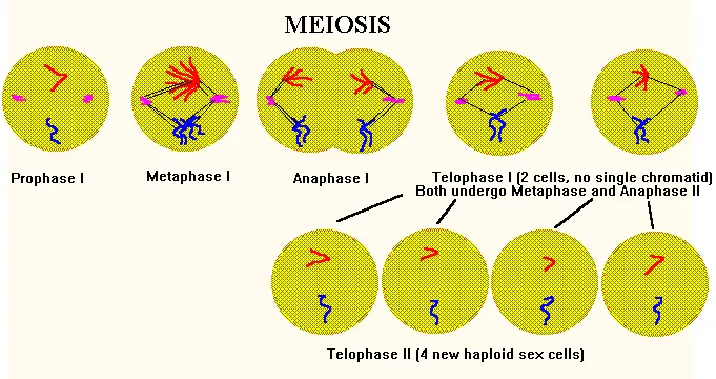
Distinguishing Features of Telophase in Meiosis:
- Chromosome Number: Meiosis encompasses two telophase stages. Following two successive rounds of chromosome segregation in meiosis I and II, telophase II culminates in the formation of haploid cells or gametes. This contrasts with mitosis, where a single telophase follows one round of chromosome segregation, yielding diploid daughter cells identical in chromosome number to the parent cell.
- Genetic Variation: A hallmark of meiosis is the generation of genetic diversity. Processes such as “crossing over” and “independent assortment” during meiosis I allow homologous chromosomes to exchange genetic segments. This genetic reshuffling ensures that the resulting daughter cells possess unique allele combinations. In contrast, mitosis, devoid of such genetic exchanges, produces daughter cells that are genetic replicas of the parent cell.
- Purpose: Telophase in meiosis is geared towards producing gametes (sperm and eggs) with a halved chromosome number, essential for sexual reproduction. Upon fertilization, these gametes merge, reinstating the diploid chromosome count in the offspring. Conversely, mitotic telophase facilitates growth, repair, and asexual reproduction by producing genetically identical daughter cells.
- Cytokinesis: In meiosis, cytokinesis is a two-fold process, occurring post both telophase I and telophase II. This bifurcation results in the parent cell’s division into four distinct haploid cells. In mitosis, however, cytokinesis transpires once, often concurrently with telophase, yielding two genetically identical diploid offspring cells.
- Centromere Dynamics: During telophase in meiosis I, the centromeres connecting sister chromatids remain intact, facilitating the separation of homologous chromosomes. This connection is pivotal for genetic material exchange between homologs. In contrast, during telophase in meiosis II, as well as in mitosis, these centromeres dissociate, leading to the segregation of sister chromatids.
In essence, telophase in meiosis, with its unique characteristics and objectives, underscores the intricacies of sexual reproduction and the perpetuation of genetic diversity, fundamental for the evolution and adaptability of species.
Telophase I
Telophase I is a pivotal stage in the meiotic process, marking the culmination of the first round of chromosome segregation. This phase is characterized by a series of intricate cellular events, each contributing to the overall objective of meiosis: ensuring genetic diversity and the formation of gametes for sexual reproduction.
Key Features of Telophase I:
- Separation of Homologous Chromosomes: One of the defining events of Telophase I is the segregation of homologous chromosomes. Unlike sister chromatids, which remain connected at this stage, homologous chromosomes move to opposite poles of the cell.
- Formation of Diploid Daughter Cells: At the conclusion of Telophase I, two diploid daughter cells are formed. It’s essential to note that while these cells are diploid, they are not genetically identical to the parent cell due to the exchange of genetic material during earlier meiotic stages.
- Potential for Genetic Variation: Telophase I is integral to the generation of genetic diversity. The separation of homologous chromosomes, combined with prior genetic exchanges, ensures that each daughter cell possesses a unique genetic composition.
- Nuclear Envelope Reformation: Contrary to some misconceptions, Telophase I witnesses the re-emergence of the nuclear envelope. This envelope encases the separated chromosomes, safeguarding the genetic material and delineating distinct nuclei within each daughter cell.
- Subsequent Cytokinesis: Following Telophase I, most organisms undergo cytokinesis, a process that divides the cell’s cytoplasm. Intriguingly, this division is often asymmetrical, resulting in one cell possessing a greater volume of cytoplasm than its counterpart. This non-uniform distribution is not arbitrary; it is crucial for the subsequent stages of meiosis and the eventual formation of viable gametes.
In summary, Telophase I in meiosis is a meticulously orchestrated phase, ensuring the accurate segregation of homologous chromosomes and laying the groundwork for the generation of genetic diversity, a cornerstone of evolution and species adaptability.
Telophase II
Telophase II, an integral stage in the meiotic process, signifies the final steps in the intricate dance of chromosome segregation. This phase is characterized by a series of cellular events, each tailored to ensure the formation of gametes, which are vital for sexual reproduction.
Principal Characteristics of Telophase II:
- Separation of Sister Chromatids: A defining event of Telophase II is the segregation of sister chromatids. These chromatids, which were previously connected at the centromere, are pulled apart and migrate to opposite poles of the cell.
- Formation of Haploid Daughter Cells: As Telophase II concludes, it paves the way for the emergence of four haploid daughter cells. These cells, commonly referred to as gametes or reproductive cells, contain half the chromosome number of the original diploid parent cell. This reduction is essential for maintaining chromosome stability across generations, as the fusion of gametes during fertilization restores the diploid state.
- Subsequent Cytokinesis: Post Telophase II, cytokinesis ensues, partitioning the cytoplasm of the two intermediate cells. This division results in the formation of four distinct haploid cells, each primed to participate in the process of fertilization.
In essence, Telophase II in meiosis is a meticulously coordinated phase, ensuring the accurate segregation of sister chromatids and facilitating the formation of haploid gametes. These gametes, bearing unique genetic compositions, are foundational for the perpetuation of genetic diversity and the continuation of life.
Difference between Telophase I and Telophase II
Telophase I vs. Telophase II
- Chromosome Number:
- Telophase I: Chromosomes consist of two sister chromatids and remain in a diploid state.
- Telophase II: Chromosomes consist of a single chromatid and are in a haploid state.
- Genetic Variation:
- Telophase I: Homologous chromosomes separate, but genetic variation is introduced due to events like crossing over in Prophase I.
- Telophase II: Sister chromatids separate, leading to four genetically distinct haploid cells.
- Nuclear Envelope:
- Telophase I: Reforms around each set of chromosomes, resulting in two nuclei in the cell.
- Telophase II: Reforms around each of the chromosomes in the four resulting cells.
- Cytokinesis:
- Telophase I: Follows Telophase I, leading to two diploid cells.
- Telophase II: Follows Telophase II, leading to four haploid cells.
- Purpose:
- Telophase I: To separate homologous chromosomes and reduce the chromosome number by half.
- Telophase II: To separate sister chromatids and produce gametes or reproductive cells.
- Centromere Connection:
- Telophase I: Centromeres of sister chromatids remain connected.
- Telophase II: Centromeres of sister chromatids separate.
| Criteria | Telophase I | Telophase II |
|---|---|---|
| Chromosome Number | Diploid number is retained | Haploid number of chromosomes |
| Chromosome Behavior | Homologous chromosomes separate | Sister chromatids separate |
| Resulting Cells | Two diploid cells | Four haploid cells |
| Nuclear Envelope | Reforms around each set of chromosomes | Reforms around individual chromatids |
| Cytokinesis | Follows, resulting in two cells | Follows, dividing each cell again |
| Genetic Variation | Crossing over has occurred | No further genetic recombination |
| Centromere Connection | Centromeres of sister chromatids remain connected | Centromeres of sister chromatids separate |
Plant Cell Telophase and Cytokinesis
Plant cells, while sharing some commonalities with animal cells during telophase and cytokinesis, exhibit distinct mechanisms and structures that cater to their unique cellular architecture and requirements.
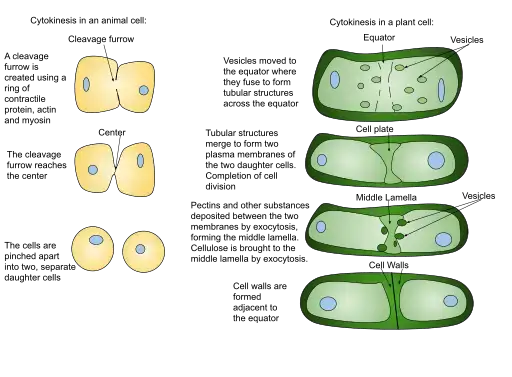
Telophase in Plant Cells:
- Chromosomal Dynamics: Similar to animal cells, plant cell telophase is characterized by the migration of chromosomes towards the cell’s opposite poles, aided by spindle fibers. Subsequently, these chromosomes undergo decondensation, transitioning back to a relaxed chromatin state.
- Centrosomal Alternatives: Unlike animal cells, plant cells lack centrosomes. Instead, they possess microtubule-organizing centers that functionally substitute centrosomes, orchestrating the assembly and organization of microtubules.
- Phragmoplast Formation: Unique to plant cells, phragmoplasts emerge during late metaphase. These structures serve as scaffolds, directing vesicles laden with cell wall materials to the cell’s equatorial region, setting the stage for cell plate formation.
Cytokinesis in Plant Cells:
- Cell Plate Formation: Distinct from the cleavage furrow mechanism observed in animal cells, plant cells undergo cytokinesis by forming a cell plate. This plate originates from the fusion of Golgi vesicles, which are rich in cellulose and other polysaccharides. These vesicles, guided by the phragmoplast, converge at the cell’s equatorial region.
- Golgi Vesicle Contribution: While the Golgi apparatus is fragmented and dispersed throughout the cytoplasm during telophase, it remains a crucial contributor to cell plate formation. The vesicles, derived from these fragmented Golgi structures, transport essential components for the cell plate. Additionally, the fragmented endoplasmic reticulum (ER) supplies vital membrane components, such as phospholipids and proteins, facilitating the expansion and fusion of Golgi vesicles during cell plate development.
- Nuclear Envelope Reformation: Concurrent with cell plate formation, the nuclear envelope in plant cells begins to reform. The membrane materials essential for this process are supplied by the ER, akin to the mechanism observed in animal cells.
- Cell Wall Formation: A defining feature of plant cell cytokinesis is the formation of cell walls. As the cell plate expands and reaches the cell’s periphery, it fuses with the existing cell wall, thereby partitioning the cell into two distinct entities.
In conclusion, telophase and cytokinesis in plant cells, while sharing foundational principles with animal cells, are tailored to accommodate the unique structural and functional needs of plants. This intricate dance of cellular events ensures the accurate segregation of genetic material and the formation of two daughter cells, each fortified with a protective cell wall.
Difference between Telophase and Cytokinesis
- Occurrence:
- Telophase: It follows Anaphase.
- Cytokinesis: It follows Telophase.
- Meaning:
- Telophase: This phase is characterized by the division of cells into two daughter nuclei.
- Cytokinesis: This phase involves the division of cells into two complete daughter cells.
- Division:
- Telophase: Only the genetic material is divided during Telophase.
- Cytokinesis: During Cytokinesis, cell organelles, cytoplasm, and the two nuclei are equally divided.
In essence, while Telophase focuses on the division and separation of genetic material into two distinct nuclei, Cytokinesis ensures the complete division of the cell, including its organelles and cytoplasm, resulting in two separate and functional daughter cells.
| Criteria | Telophase | Cytokinesis |
|---|---|---|
| Occurrence | It follows Anaphase. | It follows Telophase. |
| Meaning | This phase is characterized by the division of cells into two daughter nuclei. | This phase involves the division of cells into two complete daughter cells. |
| Division | Only the genetic material is divided during Telophase. | During Cytokinesis, cell organelles, cytoplasm, and the two nuclei are equally divided. |
Dephosphorylation of Cdk substrates
The intricate process of cell division is governed by a series of tightly regulated events, among which the dephosphorylation of mitotic cyclin-dependent kinase (Cdk) substrates during telophase plays a pivotal role. This dephosphorylation is instrumental in ensuring the precise and orderly progression of the cell cycle, particularly during the transition from mitosis to cytokinesis.
- Role of M-Cdks in Early Mitosis: M-Cdks, or Mitotic Cyclin-dependent Kinases, are crucial for the early stages of mitosis. They drive essential processes such as spindle assembly, chromosome condensation, and nuclear envelope breakdown through the phosphorylation of their protein targets.
- Transition to Telophase: As the cell progresses to telophase, the dephosphorylation of these Cdk substrates becomes imperative. This process facilitates the disassembly of the mitotic spindle, the decondensation of chromosomes, and the reformation of daughter nuclei. Achieving this transition necessitates both the inactivation of Cdks and the concurrent activation of specific phosphatases.
- Cdk Inactivation via Cyclin Degradation: The primary mechanism for Cdk inactivation is the degradation of its associated cyclin. Cyclins undergo proteolytic degradation by the anaphase promoting complex (APC) or cyclosome, a ubiquitin-ligase. Specifically, the APC bound to CDC20 (APC/CCDC20) targets mitotic cyclins for degradation, initiating this process in anaphase. Experimental evidence across various organisms, from Xenopus eggs to human cell lines, has shown that preventing M-cyclin degradation results in a cell cycle arrest between anaphase and telophase.
- Phosphatase Activation: In organisms like budding yeast, which rely on the phosphatase cdc14 for mitotic exit, the activation of this phosphatase is essential. Inhibiting cdc14 activation leads to a similar arrest in the cell cycle as blocking M-cyclin degradation.
- Cdc14 and Telophase Regulation: Cdc14 plays a central role in telophase regulation. Its activation, achieved by its release from the nucleolus and subsequent export to the cytoplasm, is crucial for telophase progression. The Cdc-14 Early Anaphase Release pathway stabilizes the spindle and releases cdc14, albeit restricted to the nucleus. Complete release and sustained activation of cdc14 are achieved by the Mitotic Exit Network (MEN) pathway, ensuring the onset of telophase events.
- Downstream Regulatory Processes: Dephosphorylation mediated by Cdc14 activates several downstream processes unique to telophase. For instance, dephosphorylation of CDH1 enables the APC/C to bind to CDH1, leading to a switch from APC/CCDC20 to APC/CCDH1 activity. This switch ensures the continued ubiquitination of mitotic cyclins and other specific targets, essential for the cell’s return to the G1 phase.
- Additional Telophase Drivers: Beyond the dephosphorylation of Cdk substrates, several other mechanisms contribute to telophase progression. Spatial cues, possibly triggered by the distancing of chromosomes from the metaphase plate during anaphase, play a role. Furthermore, cdc48 (or its human homolog, p97) is a vital regulator of telophase. This protein, through its ATPase activity, modifies proteins involved in spindle disassembly, nuclear envelope assembly, and chromosome decondensation.
In conclusion, the dephosphorylation of Cdk substrates during telophase is a critical regulatory mechanism, ensuring the orderly progression of the cell cycle and the successful division of the cell into two daughter cells.
Mitotic spindle disassembly
The mitotic spindle, a complex structure composed of microtubules and associated proteins, plays a pivotal role in ensuring the accurate segregation of chromosomes during cell division. As cells transition from anaphase-B to telophase, a defining event is the disassembly of the mitotic spindle, marking the culmination of mitosis and the onset of cellular reorganization.
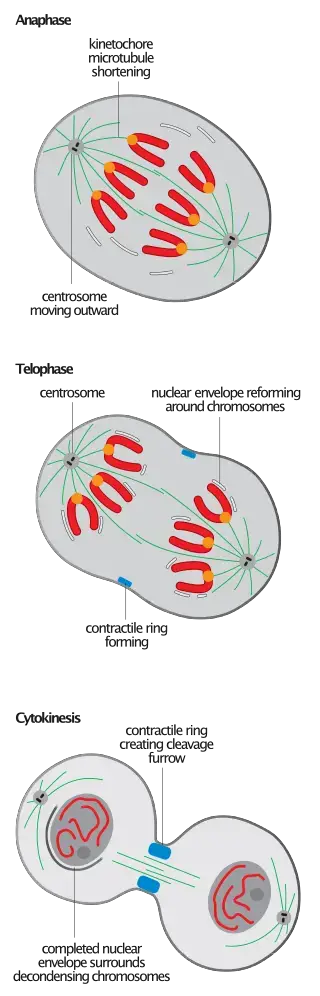
1. Characteristics of Spindle Disassembly: Spindle disassembly is an irreversible event that doesn’t lead to the ultimate degradation of its components. Instead, it results in the reorganization of the constituent microtubules. These microtubules detach from kinetochores and spindle pole bodies, reverting to their interphase configurations. The process of spindle depolymerization during telophase is essentially a reversal of spindle assembly, occurring predominantly from the microtubule’s plus end.
2. Subsequent Microtubule Reorganization: Following mitotic spindle disassembly, cells undergo a reorganization of their microtubule arrays. Unlike the polarized spindle, this assembly is interpolar. In animal cells, this reorganization is particularly evident as they form the antiparallel bundle of microtubules, known as the central spindle, which plays a crucial role in regulating cytokinesis. The ATPase p97 is instrumental in establishing the stable interphase microtubule arrays after the disassembly of the dynamic mitotic ones.
3. Molecular Mechanisms Underpinning Spindle Disassembly: While the assembly of the spindle is well-understood and is characterized by the spindle-assembly checkpoint (SAC) that oversees the formation of tentative structures, the molecular basis of spindle disassembly remains less elucidated. The dephosphorylation cascade of M-Cdk substrates, driven by the Mitotic Exit Network (MEN), is believed to orchestrate spindle disassembly. The phosphorylation states of various microtubule stabilizing and destabilizing factors, as well as microtubule nucleators, are pivotal in regulating their activities. For instance, NuMA, a Cdk substrate and a minus-end crosslinking protein, detaches from the microtubule upon its dephosphorylation during telophase.
4. A General Model for Spindle Disassembly in Yeast: In yeast, spindle disassembly can be conceptualized as a sequence of three overlapping subprocesses: spindle disengagement, destabilization, and depolymerization. These processes are orchestrated by APC/CCDH1, microtubule-stabilizer-specific kinases, and plus-end directed microtubule depolymerases, respectively. These effectors, which are conserved across eukaryotes, play distinct roles. For example, APC/CCDH1 targets microtubule-associated proteins like NuMA, Ase1, and Cin1. AuroraB phosphorylates the spindle-associated stabilizing protein EB1, causing its dissociation from microtubules, and the destabilizer She1, leading to its association with microtubules. Kinesin8, an ATP-dependent depolymerase, accelerates microtubule depolymerization at the plus end. The concurrent disruption of these mechanisms results in pronounced spindle hyperstability during telophase.
In conclusion, the disassembly of the mitotic spindle is a critical juncture in the cell division process, ensuring the smooth transition from mitosis to cytokinesis and the proper reorganization of cellular structures for the next phase of the cell cycle.
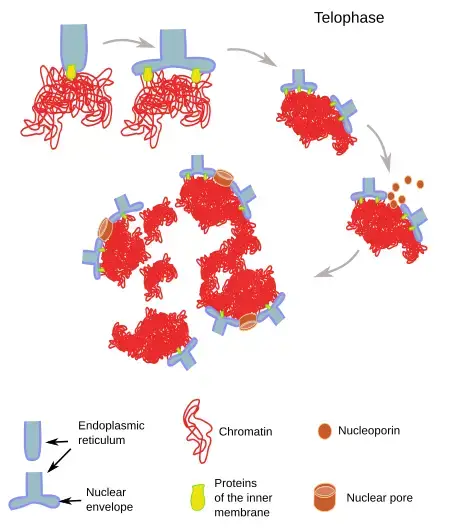
Nuclear envelope reassembly
The nuclear envelope, a pivotal cellular structure, undergoes a dynamic transformation during cell division. Its reassembly during telophase is a crucial step ensuring the proper compartmentalization of the cell’s genetic material.
- Composition and Disassembly of the Nuclear Envelope: The nuclear envelope is primarily composed of a double membrane, nuclear pore complexes, and an internal nuclear lamina adjacent to the inner nuclear membrane. During the early stages of mitosis, specifically prophase and prometaphase, this envelope is disassembled. The nuclear membrane fragments, with portions being integrated into the endoplasmic reticulum.
- Mechanism of Reassembly: During telophase, the nuclear envelope’s reformation is initiated by the aggregation of membrane-forming vesicles directly onto the chromatin surface. These vesicles then undergo lateral fusion, forming a continuous membrane. The process is facilitated by Ran-GTP, which liberates envelope components that were previously sequestered by importin β during early mitosis. Once these components are released, they can initiate the assembly of the nuclear pore complex on the chromatin surface. ELYS, a nuclear pore scaffold protein, plays a pivotal role in this process. It has the potential to recognize DNA regions rich in A:T base pairs and may bind directly to DNA or histone dimers and nucleosomes.
- Controversies Surrounding the Mechanism: The exact mechanism of nuclear envelope reassembly remains a topic of debate. Some studies suggest that the nuclear envelope primarily forms from extended ER cisternae, with nuclear pore assembly following this formation. Others propose that nuclear pore assembly precedes the recruitment of membrane vesicles around these pores.
- Role of Lamin in Nuclear Envelope Reassembly: Lamin subunits, which are disassembled during prophase, play a crucial role in the reassembly of the nuclear envelope. Their reassembly is initiated by dephosphorylation and further facilitated by methyl-esterification of COOH residues on lamin-B. While lamin-B can target chromatin as early as mid-anaphase, lamin-A’s incorporation into the nuclear envelope is a more prolonged process, extending into the G1 phase.
- Distinctions in Yeast: Interestingly, yeast, which lacks lamins, exhibits a unique behavior. Their nuclear envelope remains intact throughout mitosis, and nuclear division occurs during cytokinesis, highlighting the diverse mechanisms of nuclear envelope reassembly across different organisms.
- Research Models: The intricacies of nuclear envelope reassembly have been primarily studied using Xenopus egg extracts and human cancer cell lines, offering invaluable insights into the molecular dynamics of this process.
In conclusion, the reassembly of the nuclear envelope is a meticulously orchestrated process, ensuring the proper segregation and protection of the cell’s genetic material. Understanding this process in detail not only provides insights into cellular dynamics but also has potential implications in disease understanding and therapeutic interventions.
Chromosome decondensation
Chromosome decondensation is a pivotal cellular process that marks the transition from the mitotic phase to interphase in eukaryotic cells. This process involves the relaxation or decompaction of chromosomes, transforming them into a more expanded chromatin structure, which is essential for the resumption of interphase activities.
- Significance of Chromosome Decondensation: During cell division, chromosomes undergo a series of structural changes. They condense into tightly packed structures to ensure accurate segregation. Once this segregation is achieved, it becomes imperative for chromosomes to revert to a more relaxed state, known as decondensation. This relaxed state is crucial for the cell to resume its regular interphase processes, such as DNA replication and transcription.
- Mechanistic Insights: The Mitotic Exit Network (MEN) plays a significant role in chromosome decondensation. Specifically, the dephosphorylation mediated by MEN is essential for this process. Cyclin-dependent kinase (Cdk) dephosphorylation, orchestrated by MEN, is a critical driver of chromosome relaxation.
- Interplay with Nuclear Envelope Assembly: In many eukaryotic organisms, chromosome decondensation occurs concurrently with the reassembly of the nuclear envelope during telophase. In vertebrates, the initiation of chromosome decondensation is intricately linked to the reestablishment of nuclear import. If the transport of lamin, a crucial component of the nuclear envelope, is hindered, chromosomes remain in their condensed state even after cytokinesis. This impediment can lead to a failure in the progression to the subsequent S phase of the cell cycle.
- DNA Licensing and S Phase Entry: In mammals, the process of DNA licensing for the S phase is closely associated with chromosome decondensation. DNA licensing refers to the preparation of chromatin by associating it with specific protein factors necessary for its replication. This licensing event coincides with the maturation of the nuclear envelope during the late stages of telophase. The synchrony between these processes underscores the importance of the nuclear import machinery in reestablishing the distinct nuclear and cytoplasmic protein localizations during telophase.
Chromosome decondensation is not merely a structural transition but is deeply intertwined with the functional and regulatory aspects of cell division. Understanding this process provides insights into the intricate choreography of cellular events that ensure the proper functioning and division of a cell.
Importance of Telophase in the Cell Division and Cell Cycle Process
The cell cycle, a complex and meticulously orchestrated process, ensures the growth, development, and repair of multicellular organisms. Telophase, though brief in duration, plays a pivotal role in this cycle, particularly in the M-phase (mitotic phase), ensuring the successful culmination of cell division.
- Restoration of Cellular Structures: Telophase acts as a restorative phase, essentially reversing the cellular changes that occurred during prophase and prometaphase. During telophase, the nuclear envelope reassembles, with its membranes derived from the endoplasmic reticulum, encapsulating the separated chromatin material of the two impending daughter cells. Concurrently, new nucleoli form within each nascent nucleus, ensuring the cell’s readiness for subsequent RNA synthesis and ribosome assembly.
- Chromosomal Dynamics: Telophase oversees the crucial process of chromosomal segregation and decondensation. The condensed chromosomes, having been pulled to opposite poles of the cell, revert to their relaxed chromatin state. This transformation is vital, as chromatin is more amenable to gene expression, ensuring the genomic functionality of the daughter cells.
- Disassembly of Mitotic Apparatus: The mitotic spindle, essential for chromosome segregation in earlier mitotic stages, undergoes disassembly during telophase. This depolymerization of spindle microtubules ensures that the cell interior remains unobstructed, facilitating the subsequent processes of cytokinesis.
- Prelude to Cytokinesis: Telophase sets the stage for cytokinesis, the physical division of the cell. The successful completion of telophase ensures that the cell is primed for this final division, culminating in the formation of two genetically identical daughter cells.
- Absence of Checkpoints: While telophase lacks specific checkpoints, it is intrinsically regulated by the checkpoints of preceding phases. These earlier checkpoints ensure the accurate progression of the cell cycle, and by the time telophase is reached, the cell has effectively completed the essential steps of mitosis.
- Regulation by Cdk Substrate Dephosphorylation: The progression of telophase is driven by the dephosphorylation of Cdk (cyclin-dependent kinase) substrates. This process, which involves the removal of phosphate groups from proteins, leads to the disassembly of the mitotic spindle, chromosome decondensation, formation of new nuclear envelopes, and the establishment of two distinct nuclei in the daughter cells.
In conclusion, telophase, though brief, is indispensable in the cell division process. It ensures the accurate segregation of genetic material, restoration of cellular structures, and prepares the cell for its final division, underscoring its paramount importance in the cell cycle.
Significance of Telophase
Telophase, one of the concluding stages of mitosis and meiosis, plays a pivotal role in ensuring the successful completion of cell division. Its significance can be understood by examining its primary functions and outcomes:
- Completion of Chromosomal Segregation:
- Telophase ensures that the chromosomes, which have been separated during anaphase, are fully segregated into two distinct sets, each set being enclosed within a newly forming nucleus. This ensures that each daughter cell receives an exact copy of the genetic material.
- Reformation of the Nuclear Envelope:
- One of the hallmarks of telophase is the reformation of the nuclear envelope around the separated sets of chromosomes. This re-establishes the nuclear compartment, separating the genetic material from the cytoplasm.
- Chromosome Decondensation:
- Chromosomes, which were condensed to ensure proper segregation during earlier stages of mitosis, begin to decondense or relax during telophase. This decondensation is crucial for the cell to resume its regular interphase processes, such as DNA replication and transcription.
- Restoration of Nucleoli:
- The nucleoli, which disintegrate during prophase, begin to reappear in telophase. The nucleolus is essential for ribosomal RNA synthesis and ribosome assembly, crucial components for protein synthesis.
- Setting the Stage for Cytokinesis:
- Telophase prepares the cell for the final step of cell division – cytokinesis. By the end of telophase, the cell is ready to physically divide its cytoplasm, ensuring that each daughter cell not only gets an accurate copy of the genetic material but also receives the necessary cellular organelles and cytoplasmic components.
- Ensuring Genetic Stability:
- By ensuring that each daughter cell receives a complete set of chromosomes, telophase plays a crucial role in maintaining genetic stability across cell generations. Any errors in this process could lead to genetic abnormalities or diseases.
- Facilitating Genetic Diversity (in Meiosis):
- In the context of meiosis, which leads to the formation of gametes for sexual reproduction, telophase is essential for producing cells with half the chromosome number of the parent cell. This reduction is crucial for maintaining the chromosome number of a species when gametes fuse during fertilization.
In conclusion, telophase is not just a transitional phase but a culmination of a series of meticulously orchestrated events that ensure the continuity and stability of life at the cellular level. Its significance lies in its role in safeguarding the integrity of genetic information and facilitating the propagation of life.
Quiz
Which of the following best describes the primary event during telophase?
a) Chromosomes condense
b) Nuclear envelope disintegrates
c) Chromosomes align at the metaphase plate
d) Nuclear envelope reforms around separated chromosomes
[expand title=”Show answer” swaptitle=”Hide answer”] Answer: d) Nuclear envelope reforms around separated chromosomes [/expand]
During which phase of mitosis do chromosomes begin to decondense or relax?
a) Prophase
b) Metaphase
c) Anaphase
d) Telophase
[expand title=”Show answer” swaptitle=”Hide answer”] Answer: d) Telophase [/expand]
In telophase, the nucleoli:
a) Disintegrate
b) Remain unchanged
c) Begin to reappear
d) Align at the metaphase plate
[expand title=”Show answer” swaptitle=”Hide answer”] Answer: c) Begin to reappear [/expand]
Which of the following is NOT a characteristic of telophase?
a) Spindle fibers disintegrate
b) Chromosomes segregate to opposite poles
c) Nuclear envelope breaks down
d) Chromosomes decondense
[expand title=”Show answer” swaptitle=”Hide answer”] Answer: c) Nuclear envelope breaks down [/expand]
Telophase prepares the cell for which subsequent phase of cell division?
a) Prophase
b) Metaphase
c) Cytokinesis
d) Interphase
[expand title=”Show answer” swaptitle=”Hide answer”] Answer: c) Cytokinesis [/expand]
In which phase of meiosis does the nuclear envelope reform around haploid sets of chromosomes?
a) Telophase I
b) Telophase II
c) Anaphase I
d) Anaphase II
[expand title=”Show answer” swaptitle=”Hide answer”] Answer: b) Telophase II [/expand]
Which organelle plays a role in the reformation of the nuclear envelope during telophase?
a) Golgi apparatus
b) Mitochondria
c) Endoplasmic reticulum
d) Lysosome
[expand title=”Show answer” swaptitle=”Hide answer”] Answer: c) Endoplasmic reticulum [/expand]
Which of the following is true about telophase in plant cells?
a) A cleavage furrow forms
b) A cell plate begins to form
c) Centrosomes play a major role
d) Chromosomes remain condensed
[expand title=”Show answer” swaptitle=”Hide answer”] Answer: b) A cell plate begins to form [/expand]
Which protein complex is responsible for chromosome decondensation during telophase?
a) Cyclin A
b) Cyclin B
c) Cyclin D
d) Cyclin E
[expand title=”Show answer” swaptitle=”Hide answer”] Answer: b) Cyclin B [/expand]
Which of the following is NOT a function of telophase?
a) Chromosome segregation
b) Spindle fiber formation
c) Nuclear envelope reformation
d) Chromosome decondensation
[expand title=”Show answer” swaptitle=”Hide answer”] Answer: b) Spindle fiber formation [/expand]
FAQ
What is telophase in cell division?
Telophase is the final phase of mitosis and meiosis where the separated chromosomes are enclosed by a nuclear envelope, resulting in the formation of two nuclei within one cell.
How does telophase differ in mitosis and meiosis?
In mitosis, telophase results in two diploid daughter nuclei within a single cell. In meiosis, telophase occurs twice (telophase I and II), with telophase I leading to two diploid nuclei and telophase II resulting in four haploid nuclei.
What are the key events during telophase?
The main events include the decondensation of chromosomes, reformation of the nuclear envelope around each set of chromosomes, reformation of nucleoli, and the disassembly of the spindle apparatus.
Why is telophase important in the cell cycle?
Telophase is crucial as it ensures the proper segregation of genetic material into two distinct nuclei, preparing the cell for cytokinesis, which will physically divide the cell into two.
How does telophase contribute to genetic diversity?
While telophase itself doesn’t directly contribute to genetic diversity, it follows earlier stages of meiosis where genetic recombination occurs. Telophase ensures that the recombined genetic material is properly enclosed in separate nuclei.
What happens to the chromosomes during telophase?
Chromosomes, which were previously condensed and aligned for segregation, begin to decondense or relax back into a more extended chromatin state during telophase.
How does telophase differ in plant and animal cells?
In both, the nuclear envelope reforms during telophase. However, in plant cells, a cell plate begins to form in the middle of the cell, which will eventually become the cell wall. In animal cells, a cleavage furrow forms to divide the cell.
What follows telophase in the cell cycle?
Cytokinesis follows telophase, which is the process where the cytoplasm of the cell is divided, resulting in two separate daughter cells.
How is the nuclear envelope reformed in telophase?
The nuclear envelope reforms around the chromosomes from vesicles of the endoplasmic reticulum and other fragments of the original nuclear envelope.
Are the daughter cells produced after telophase genetically identical?
In mitosis, the daughter cells are genetically identical to the parent cell. In meiosis, due to genetic recombination events earlier in the process, the daughter cells are genetically unique.
References
- Biology Dictionary. (Year). Telophase. Retrieved from https://biologydictionary.net/telophase/
- Biology Online. (Year). Telophase. Retrieved from https://www.biologyonline.com/dictionary/telophase
- Wikipedia. (Year). Telophase. Retrieved from https://en.wikipedia.org/wiki/Telophase
- Toppr. (Year). Telophase: Definition and Explanation. Retrieved from https://www.toppr.com/guides/biology/cell-the-unit-of-life/telophase-definition-and-explanation/
- Study.com. (Year). Telophase in Mitosis: Definition & Overview. Retrieved from https://study.com/learn/lesson/telophase-mitosis.html
- SparkNotes. (Year). Mitosis: Telophase and Cytokinesis. Retrieved from https://www.sparknotes.com/biology/cellreproduction/mitosis/section3/
- Johnson, D. G., & Walker, C. L. (1999). Cyclins and cell cycle checkpoints. Annual Review of Pharmacology and Toxicology, 39.
- Bower, J.J., Vance, L.D., Psioda, M., & et al. (2017). Patterns of cell cycle checkpoint deregulation associated with intrinsic molecular subtypes of human breast cancer cells. npj Breast Cancer, 3(9). https://doi.org/10.1038/s41523-017-0009-7
- Israels, E. D., & Israels, L. G. (2000). The cell cycle. The Oncologist, 5(6), 510-513.
- Schafer, K. A. (1998). The cell cycle: a review. Veterinary Pathology, 35(6), 461-478.
- Knoblich, J. A. (2010). Asymmetric cell division: recent developments and their implications for tumour biology. Nature Reviews Molecular Cell Biology, 11(12), 849-860.
- Debatisse, M., & Rosselli, F. (2019). A journey with common fragile sites: From S phase to telophase. Genes, Chromosomes and Cancer, 58(5), 305-31