The SXT test is a laboratory antimicrobial susceptibility test that is used to determine the sensitivity or resistance of bacteria to a combined antibiotic preparation of sulfamethoxazole and trimethoprim (SXT). It is the process in which a paper disc impregnated with fixed concentration of these two drugs is placed on a culture medium previously inoculated with the test organism. These two antibiotics act synergistically and inhibit the sequential steps of folic acid synthesis in bacteria which is essential for synthesis of nucleic acids and proteins.
It is based on the principle that sulfamethoxazole blocks the conversion of para-aminobenzoic acid to dihydrofolic acid while trimethoprim inhibits the reduction of dihydrofolic acid to tetrahydrofolic acid. Due to this combined action the bacterial growth is inhibited effectively. After incubation the plate is observed for zone of inhibition around the SXT disc. The presence of a clear zone indicates susceptibility whereas absence of zone indicates resistance of the organism.
Apart from its therapeutic importance the SXT test is also used for presumptive identification of certain streptococci. It is helpful in differentiating Group A and Group B streptococci which are resistant to SXT from other beta-hemolytic streptococci such as Group C, F and G which are susceptible. Thus the SXT test is commonly employed in routine microbiology laboratories both for antibiotic susceptibility testing and for supporting identification of bacterial isolates.
Objectives of SXT (Sulfamethoxazole-Trimethoprim) Test
- To determine the susceptibility or resistance of bacteria to sulfamethoxazole and trimethoprim for selection of suitable antimicrobial therapy.
- To differentiate Group A and Group B beta-hemolytic streptococci which are resistant to SXT from other streptococcal groups which are susceptible.
- To help in presumptive identification of streptococci when used along with other tests such as bacitracin sensitivity.
- To aid in isolation of Group A and Group B streptococci from mixed clinical specimens by inhibiting normal commensal flora.
- To monitor resistance pattern of organisms like Vibrio cholerae during epidemiological investigations and outbreaks.
Principle of SXT (sulfamethoxazole-trimethoprim) Test
The principle of SXT test is based on the combined and synergistic action of two antimicrobial agents, sulfamethoxazole and trimethoprim, which inhibit the bacterial folic acid synthesis pathway. Sulfamethoxazole competes with para-aminobenzoic acid (PABA) and inhibits the enzyme dihydropteroate synthase, whereas trimethoprim inhibits the enzyme dihydrofolate reductase. Due to this sequential inhibition the formation of tetrahydrofolic acid is prevented which is required for synthesis of nucleic acids and proteins in bacteria. As a result the bacterial growth is inhibited and multiplication of cells is stopped.
In laboratory conditions this principle is demonstrated by the disc diffusion method. A disc impregnated with sulfamethoxazole-trimethoprim is placed on agar medium inoculated with the test organism. The antibiotics diffuse into the surrounding medium and create a concentration gradient. If the organism is susceptible growth is inhibited around the disc forming a clear zone of inhibition whereas resistant organisms grow up to the edge of the disc. This characteristic pattern is also useful in differentiation of beta-hemolytic streptococci where Group A and Group B streptococci show resistance while Groups C and G remain susceptible.
Materials Required for SXT Test
- SXT antibiotic disc containing sulfamethoxazole (23.75 µg) and trimethoprim (1.25 µg) in fixed proportion.
- Culture media such as Mueller-Hinton agar poured to standard thickness for routine organisms.
- Mueller-Hinton agar supplemented with 5% blood or defibrinated blood agar for fastidious organisms like streptococci.
- Media with low thymidine content or media containing thymidine phosphorylase to avoid false resistance result.
- Pure culture of test organism which is 18–24 hours old.
- Bacterial suspension adjusted to 0.5 McFarland turbidity standard.
- Sterile cotton swab for uniform inoculation of the agar surface.
- Sterile forceps or disc dispenser for placement of SXT disc.
- Incubator maintained at 35°C ± 2°C for proper incubation.
- CO₂ enriched atmosphere (5–10%) for streptococcal species.
- Standard control strains such as Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 25923 for quality control.
- Measuring scale or caliper for measurement of zone of inhibition in millimeter.
Procedure of SXT (sulfamethoxazole-trimethoprim) Test
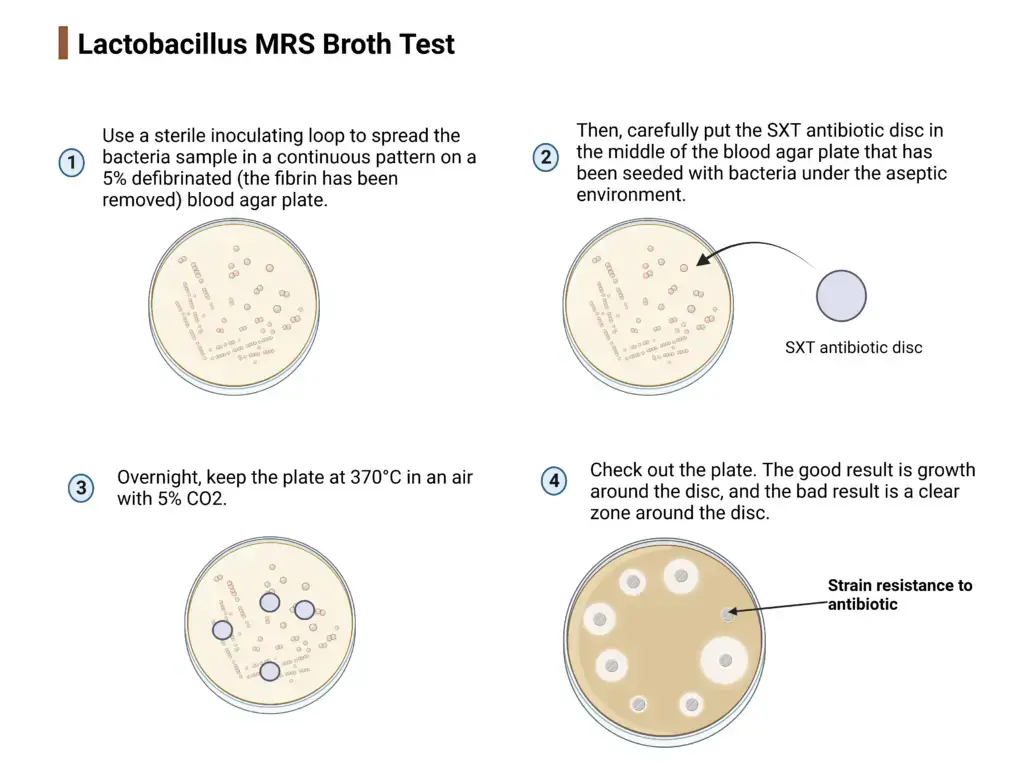
The procedure of SXT test is carried out to determine the susceptibility or resistance of organisms to sulfamethoxazole-trimethoprim antibiotic. It is commonly performed by disc diffusion method under laboratory conditions.
Procedure
- Preparation of inoculum
- A pure culture of the test organism is taken.
- The bacterial suspension is prepared in sterile saline or broth.
- The turbidity of the suspension is adjusted to 0.5 McFarland standard.
- Inoculation of media
- A sterile swab is dipped into the standardized inoculum.
- Excess fluid is removed by pressing the swab against the wall of tube.
- The swab is streaked evenly over the entire surface of agar plate (Blood agar or Mueller Hinton agar).
- The plate is rotated and streaking is repeated to obtain uniform lawn culture.
- Drying of plate
- The inoculated agar plate is allowed to stand for 3–5 minutes.
- This step helps in absorption of excess moisture from surface.
- Placement of SXT disc
- The SXT disc (1.25 µg trimethoprim + 23.75 µg sulfamethoxazole) is placed on agar surface using sterile forceps.
- The disc is gently pressed to ensure proper contact with medium.
- If required, other antibiotic discs may be placed at proper distance.
- Incubation
- The plates are incubated in inverted position.
- Incubation is done at 35–37°C for 18–24 hours.
- For streptococci, incubation is done in presence of 5–10% CO₂.
- Observation of result
- After incubation, the plate is examined for zone of inhibition around the SXT disc.
- The diameter of zone is measured in millimeters.
- Interpretation
- Presence of clear zone indicates susceptibility of organism.
- Absence of zone or growth up to disc indicates resistance.
- The result is interpreted using standard chart for reporting.
Result of SXT Test
The result of SXT test is observed by the presence or absence of zone of inhibition around the SXT disc. The interpretation is done based on the purpose of test.
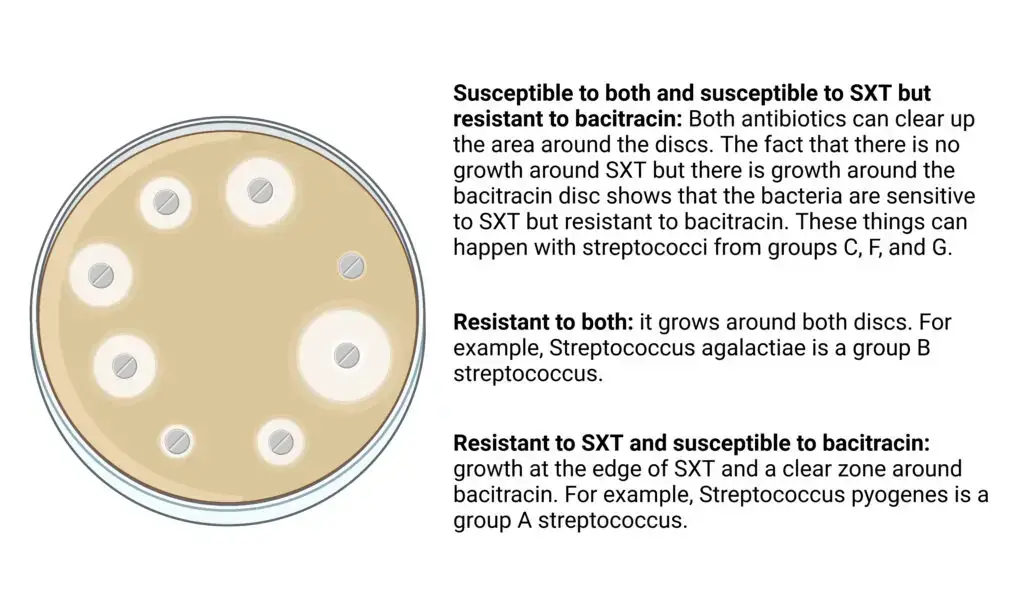
Result for presumptive identification of beta-hemolytic Streptococci
- Susceptible
- A clear zone of inhibition is seen around the SXT disc.
- This indicates that the organism is sensitive to SXT.
- The organism is likely Group C, Group F or Group G streptococci.
- Resistant
- Growth of organism is seen up to the edge of SXT disc.
- No clear zone of inhibition is observed.
- The organism is likely Group A (Streptococcus pyogenes) or Group B (Streptococcus agalactiae).
- To differentiate Group A and Group B, Bacitracin disc is used where Group A is sensitive and Group B is resistant.
Result for antibiotic susceptibility testing (Kirby–Bauer method)
- Susceptible (S)
- Zone of inhibition is ≥ 16 mm.
- The organism is inhibited by sulfamethoxazole-trimethoprim.
- The antibiotic can be used for treatment.
- Intermediate (I)
- Zone of inhibition is between 11–15 mm.
- The organism shows intermediate response to antibiotic.
- The drug may act at higher concentration or specific site.
- Resistant (R)
- Zone of inhibition is ≤ 10 mm.
- The organism is not inhibited by the antibiotic.
- Treatment with SXT is ineffective.
Important points while reading result
- Slight hazy growth within the zone is ignored (up to 20%).
- Only the clear margin of inhibition is measured.
- In Proteus species, swarming growth is ignored and outer clear zone is measured.
Quality control of SXT (Sulfamethoxazole–Trimethoprim) Test
Quality control is done to ensure accuracy of SXT susceptibility result. Mueller Hinton agar used for test should contain low level of thymidine and thymine.
- Control strain for thymidine content
- Enterococcus faecalis ATCC 29212 is used.
- Zone of inhibition should be ≥ 20 mm.
- Zone less than 20 mm or growth within zone indicates unsuitable media.
- Control strains for disc potency and method validation
- Escherichia coli ATCC 25922 – zone size should be within standard range.
- Staphylococcus aureus ATCC 25923 – zone size should be within standard range.
- These strains confirm proper diffusion and potency of SXT disc.
- Quality control should be performed with each new batch of media and antibiotic discs.
- Media parameters
- pH of agar should be between 7.2–7.4.
- Agar depth should be about 4 mm.
- Incorrect pH or depth may alter zone size.
- Incubation conditions
- Plates are incubated at 35–37°C.
- Incubation in CO₂ should be avoided as it may affect pH and result.
- Quality control for SXT blood agar
- Group A or Group B streptococci should show growth.
- Normal flora organisms should be inhibited.
- Only when all quality control parameters are satisfactory, SXT test results are reported.
Uses of SXT (Sulfamethoxazole–Trimethoprim) Test
- It is used for presumptive identification of beta-hemolytic streptococci.
- It is used to differentiate Group A and Group B streptococci from Group C, F and G streptococci.
- It is used along with Bacitracin (Taxo A) test for better identification of streptococcal groups.
- It is used for selective isolation of Group A and Group B streptococci by inhibiting normal flora on blood agar.
- It is used for antibiotic susceptibility testing by Kirby–Bauer disc diffusion method.
- It is used to determine susceptibility of organisms like E. coli, Staphylococcus and MRSA to SXT.
- It is used to guide treatment and prophylaxis of Pneumocystis carinii pneumonia.
- It is used in epidemiological studies to monitor drug resistance patterns, especially in Vibrio cholerae.
Advantages of SXT Test
- It is used for presumptive identification of beta-hemolytic streptococci.
- It helps in differentiation of Group A and Group B streptococci from Group C, F and G streptococci.
- It helps in selective recovery of Group A and Group B streptococci by inhibiting normal throat flora.
- It shows synergistic action as sulfamethoxazole and trimethoprim block two successive steps of folic acid synthesis.
- It reduces the rate of development of bacterial resistance as compared to single drug testing.
- It is useful for epidemiological monitoring of drug resistance, especially in Vibrio cholerae.
- It is simple, economical and useful in laboratories where serological grouping facilities are not available.
Limitations of SXT (Sulfamethoxazole–Trimethoprim) Test
- Presence of thymidine or thymine in the media may interfere with test result and give false resistance.
- Improper blood supplementation in agar media may affect the activity of SXT and alter zone size.
- Zone of inhibition often shows trailing or hazy growth which makes reading of result difficult.
- Interpretation of zone is subjective as slight growth inside zone has to be ignored (80% rule).
- It gives only presumptive identification of streptococci and not confirmatory result.
- The test should be used along with Bacitracin or other tests for accurate identification.
- Due to widespread resistance, it is no longer reliable for differentiation of Vibrio cholerae strains.
- Variation in agar depth may lead to false susceptible or resistant result.
- Heavy inoculum may reduce zone size and produce false resistance.
- Delay in disc placement or incubation affects diffusion of antibiotic and result.
- Incubation in CO₂ may change pH of medium and influence the potency of SXT.
- American Society for Microbiology. (2009). Kirby-Bauer disk diffusion susceptibility test protocol (J. Hudzicki, Author). https://asm.org/getattachment/2594ce26-bd44-47f6-8287-0657aa9185ad/kirby-bauer-disk-diffusion-susceptibility-test-protocol-pdf.pdf
- American Society for Microbiology. (2011). A practical guidance document for the laboratory detection of Vibrio cholerae. https://asm.org/asm/media/policy-and-advocacy/vibriocholerae1-11-11.pdf
- ATCC. (n.d.). Enterococcus faecalis (Andrewes and Horder) Schleifer and Kilpper-Balz – 29212-MINI-PACK. https://www.atcc.org/products/29212-mini-pack
- Baron, S., Larvor, E., Chevalier, S., Jouy, E., Kempf, I., Granier, S. A., & Lesne, J. (2017). Antimicrobial susceptibility among urban wastewater and wild shellfish isolates of non-O1/non-O139 Vibrio cholerae from La Rance Estuary (Brittany, France). Frontiers in Microbiology, 8, 1637. https://doi.org/10.3389/fmicb.2017.01637
- Biology LibreTexts. (2024, February 6). 9: Kirby-Bauer (antibiotic sensitivity). https://bio.libretexts.org/Learning_Objects/Laboratory_Experiments/Microbiology_Labs/Microbiology_Labs_I/09%3A_Kirby-Bauer_(Antibiotic_Sensitivity)
- Bowen, A. C., Carapetis, J. R., Currie, B. J., Fowler, V., Jr., Chambers, H. F., & Tong, S. Y. C. (2017). Sulfamethoxazole-trimethoprim (cotrimoxazole) for skin and soft tissue infections including impetigo, cellulitis, and abscess. Open Forum Infectious Diseases, 4(4), ofx232. https://doi.org/10.1093/ofid/ofx232
- Centers for Disease Control and Prevention. (2024, July). Chapter 6: Isolation and identification of Vibrio cholerae serogroups O1 and O139. https://www.cdc.gov/cholera/media/pdfs/2024/07/Chapter-6-Laboratory-methods-for-the-diagnosis-of-epidemic-dysentery-and-cholera_ENG-6.pdf
- Clinical and Laboratory Standards Institute. (2016). M45: Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria (3rd ed.). https://clsi.org/shop/standards/m45/
- Dalynn Biologicals. (2014, October). Bacitracin differentiation disks (DB10). https://www.dalynn.com/dyn/ck_assets/files/tech/DB10.pdf
- England, M., & Clark, A. (2025, March 26). SCACM workshop: Antimicrobial susceptibility testing. South Central Association for Clinical Microbiology. https://scacm.org/2025_Spring/Handouts/362-112-25-1perPg_HANDOUT-AST_Workshop.pdf
- Fosun Pharma USA Inc. (2019, September). Sulfamethoxazole and trimethoprim (double strength) tablets, USP. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/018598s052lbl.pdf
- Global Task Force on Cholera Control. (2023). Antimicrobial susceptibility testing for treatment and control of cholera. https://www.gtfcc.org/wp-content/uploads/2025/03/gtfcc-job-aid-antimicrobial-susceptibility-testing-for-treatment-and-control-of-cholera-en.pdf
- Gunn, B. A. (1976). SXT and Taxo A disks for presumptive identification of group A and B streptococci in throat cultures. Journal of Clinical Microbiology, 4(2), 192–193. https://doi.org/10.1128/jcm.4.2.192-193.1976
- Hardy Diagnostics. (2024, February 5). How to do a Kirby Bauer disk diffusion antimicrobial susceptibility test. https://hardydiagnostics.com/blog/how-to-do-a-kirby-bauer-disk-diffusion-antimicrobial-susceptibility-test
- Hombach, M., Zbinden, R., & Böttger, E. C. (2013). Standardisation of disk diffusion results for antibiotic susceptibility testing using the sirscan automated zone reader. BMC Microbiology, 13, 225. https://doi.org/10.1186/1471-2180-13-225
- Jain, M., Kushwah, K. S., Kumar, P., & Goel, A. K. (2013). Molecular characterization of Vibrio cholerae O1 reveals continuous evolution of its new variants in India. Indian Journal of Microbiology, 53(2), 137–141. https://doi.org/10.1007/s12088-013-0372-5
- Jones, C., Stevens, D. L., & Ojo, O. (1987). Effect of minimal amounts of thymidine on activity of trimethoprim-sulfamethoxazole against Staphylococcus epidermidis. Antimicrobial Agents and Chemotherapy, 31(2), 144–147. https://doi.org/10.1128/aac.31.2.144
- Kiehlbauch, J. A., Hannett, G. E., Salfinger, M., Archinal, W., Monserrat, C., & Carlyn, C. (2000). Use of the National Committee for Clinical Laboratory Standards guidelines for disk diffusion susceptibility testing in New York State laboratories. Journal of Clinical Microbiology, 38(9), 3341–3348. https://doi.org/10.1128/jcm.38.9.3341-3348.2000
- Lesmana, M., Albert, M. J., Subekti, D., Richie, E., Tjaniadi, P., Walz, S. E., & Lebron, C. I. (1996). Simple differentiation of Vibrio cholerae O139 from V. cholerae O1 and non-O1, non-O139 by modified CAMP test. Journal of Clinical Microbiology, 34(4), 1038–1040. https://doi.org/10.1128/jcm.34.4.1038-1040.1996
- Micromaster Laboratories. (2018). Bacitracin (0.04 Units) (ID001) – Product specification sheet. https://www.micromasterlab.com/wp-content/uploads/bsk-pdf-manager/ID001_Bacitracin_Susceptibility_Test_Disc-PSS_1802.pdf
- Minato, Y., Dawadi, S., Kordus, S. L., Sivanandam, A., Aldrich, C. C., & Baughn, A. D. (2018). Mutual potentiation drives synergy between trimethoprim and sulfamethoxazole. Nature Communications, 9, 1003. https://doi.org/10.1038/s41467-018-03447-x
- Mukhopadhyay, A. K., Garg, S., Nair, G. B., Kar, S., Ghosh, R. K., Pajni, S., Ghosh, A., Shimada, T., Takeda, T., & Takeda, Y. (1995). Biotype traits and antibiotic susceptibility of Vibrio cholerae serogroup O1 before, during and after the emergence of the O139 serogroup. Epidemiology and Infection, 115(3), 427–434. https://doi.org/10.1017/s0950268800058581
- Okoh, A. I., & Igbinosa, E. O. (2010). Antibiotic susceptibility profiles of some Vibrio strains isolated from wastewater final effluents in a rural community of the Eastern Cape Province of South Africa. BMC Microbiology, 10, 143. https://doi.org/10.1186/1471-2180-10-143
- Pande, K., Mendiratta, D. K., Vijayashri, D., Thamke, D. C., & Narang, P. (2012). SXT constin among Vibrio cholerae isolates from a tertiary care hospital. The Indian Journal of Medical Research, 135(3), 346–350.
- Patsnap Synapse. (2023). What is the mechanism of folic acid? https://synapse.patsnap.com/article/what-is-the-mechanism-of-folic-acid
- Patsnap Synapse. (2023). What is the mechanism of trimethoprim? https://synapse.patsnap.com/article/what-is-the-mechanism-of-trimethoprim
- Rapid Microbiology. (n.d.). Vibrio species detection and identification in seafood. https://www.rapidmicrobiology.com/test-method/detection-and-identification-of-vibrio-species-in-food
- ResearchGate. (n.d.). Summarized pathway of folic acid metabolism, including bacterial de novo synthesis, reduction and TS-mediated feedback loop [Figure]. https://www.researchgate.net/figure/Summarized-pathway-of-folic-acid-metabolism-including-bacterial-de-novo-synthesis_fig2_336405765
- Sciortino, C. V., Johnson, J. A., & Hamad, A. (1996). Vitek system antimicrobial susceptibility testing of O1, O139, and non-O1 Vibrio cholerae. Journal of Clinical Microbiology, 34(4), 897–900. https://doi.org/10.1128/jcm.34.4.897-900.1996
- Shrestha, A. (n.d.). SXT testing: Principle, procedure, and results. Microbe Online. https://microbeonline.com/sxt-testing-principle-procedure-and-results/
- Siegrist, J. (n.d.). Streptococci – Overview of detection, identification, differentiation and cultivation techniques. Sigma-Aldrich. https://www.sigmaaldrich.com/US/en/technical-documents/technical-article/cell-culture-and-cell-culture-analysis/mammalian-cell-culture/streptococci-overview-of-detection
- Sigma-Aldrich. (n.d.). Folic acid metabolism in cancer. https://www.sigmaaldrich.com/US/en/technical-documents/technical-article/research-and-disease-areas/cancer-research/folic-acid-metabolism
- Spellerberg, B., & Brandt, C. (2016). Laboratory diagnosis of Streptococcus pyogenes (group A streptococci). In J. J. Ferretti, D. L. Stevens, & V. A. Fischetti (Eds.), Streptococcus pyogenes: Basic biology to clinical manifestations. University of Oklahoma Health Sciences Center. https://www.ncbi.nlm.nih.gov/books/NBK343617/
- Stoner, R. A. (1978). Bacitracin and coagglutination for grouping of beta-hemolytic streptococci. Journal of Clinical Microbiology, 7(5), 463–466. https://doi.org/10.1128/jcm.7.5.463-466.1978
- Tabatabaei, S. M., & Khorashad, A. S. (2015). Antimicrobial resistance patterns of Vibrio cholera strains isolated from Afghan and Iranian patients in Iran. International Journal of Infection, 2(1), e22822. https://doi.org/10.17795/iji-22822
- Wikipedia. (2025, February 24). Disk diffusion test. https://en.wikipedia.org/wiki/Disk_diffusion_test
- Zander, J., Besier, S., Ackermann, H., & Wichelhaus, T. A. (2010). Synergistic antimicrobial activities of folic acid antagonists and nucleoside analogs. Antimicrobial Agents and Chemotherapy, 54(3), 1226–1231. https://doi.org/10.1128/AAC.00705-09