What is Sterilization?
- Sterilization is a critical process in microbiology and medical practice designed to eliminate all forms of life and biological agents from surfaces, objects, or fluids. This process ensures that no viable microorganisms, including bacteria, fungi, spores, viruses, or prions, remain.
- Sterilization encompasses a variety of methods. Heat is one of the most common techniques, utilizing high temperatures to destroy microorganisms. Autoclaving, which involves steam under pressure, is a typical example of heat-based sterilization. Chemical agents, such as ethylene oxide or hydrogen peroxide, offer another route by disrupting microbial cellular processes and structures.
- Irradiation, using ultraviolet light or gamma rays, can also achieve sterilization by causing DNA damage in microorganisms. High pressure, through techniques such as pressure cooking, inactivates microbial life by altering cellular structures. Filtration, often used for liquids, physically removes microorganisms from solutions.
- It is essential to distinguish sterilization from related processes such as disinfection, sanitization, and pasteurization. While these methods reduce the number of microorganisms, they do not eliminate all forms of life. Disinfection reduces microbial populations to levels considered safe, sanitization decreases them to acceptable levels, and pasteurization targets specific pathogens without achieving complete sterility.
- In summary, sterilization is defined by its ability to eradicate all forms of life and biological agents. The choice of method depends on the specific requirements of the situation, including the nature of the material being sterilized and the type of microorganisms targeted.
Important Definitions
- Sterilization
- Definition: Sterilization is a process designed to eliminate all microorganisms, including both vegetative cells and spores, from an object, surface, or medium.
- Purpose: It ensures complete microbial eradication, rendering the object or surface sterile and free of all forms of life.
- Methods: Techniques include heat, chemicals, irradiation, high pressure, and filtration.
- Disinfection
- Definition: Disinfection involves the destruction of pathogenic microorganisms that can cause infection, though it does not necessarily kill all spores.
- Purpose: The aim is to reduce the number of microorganisms to levels that are not harmful to health.
- Scope: While disinfection significantly decreases microbial presence, it does not achieve the complete microbial eradication associated with sterilization.
- Antiseptics
- Definition: Antiseptics are chemical agents applied to living tissues to prevent infection by inhibiting the growth of microorganisms.
- Purpose: These substances are used to prevent infection and control microbial growth on skin or mucous membranes.
- Characteristics: Antiseptics must be safe for application on living tissues and effective against a range of microorganisms.
- Asepsis
- Definition: Asepsis refers to techniques and practices aimed at preventing infection in uninfected tissues or environments.
- Purpose: The goal is to avoid the introduction of pathogens into sterile areas or materials.
- Methods: Aseptic techniques include sterilization procedures, maintaining sterile fields, and using protective barriers to prevent contamination.
Methods of Sterilization
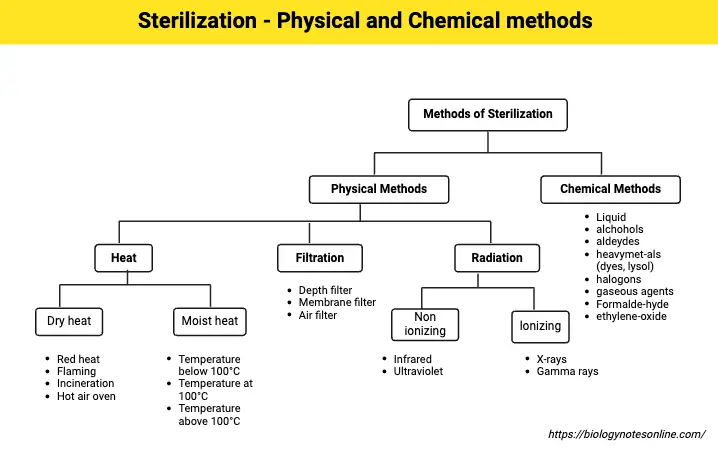
A. Physical Methods of Sterilization
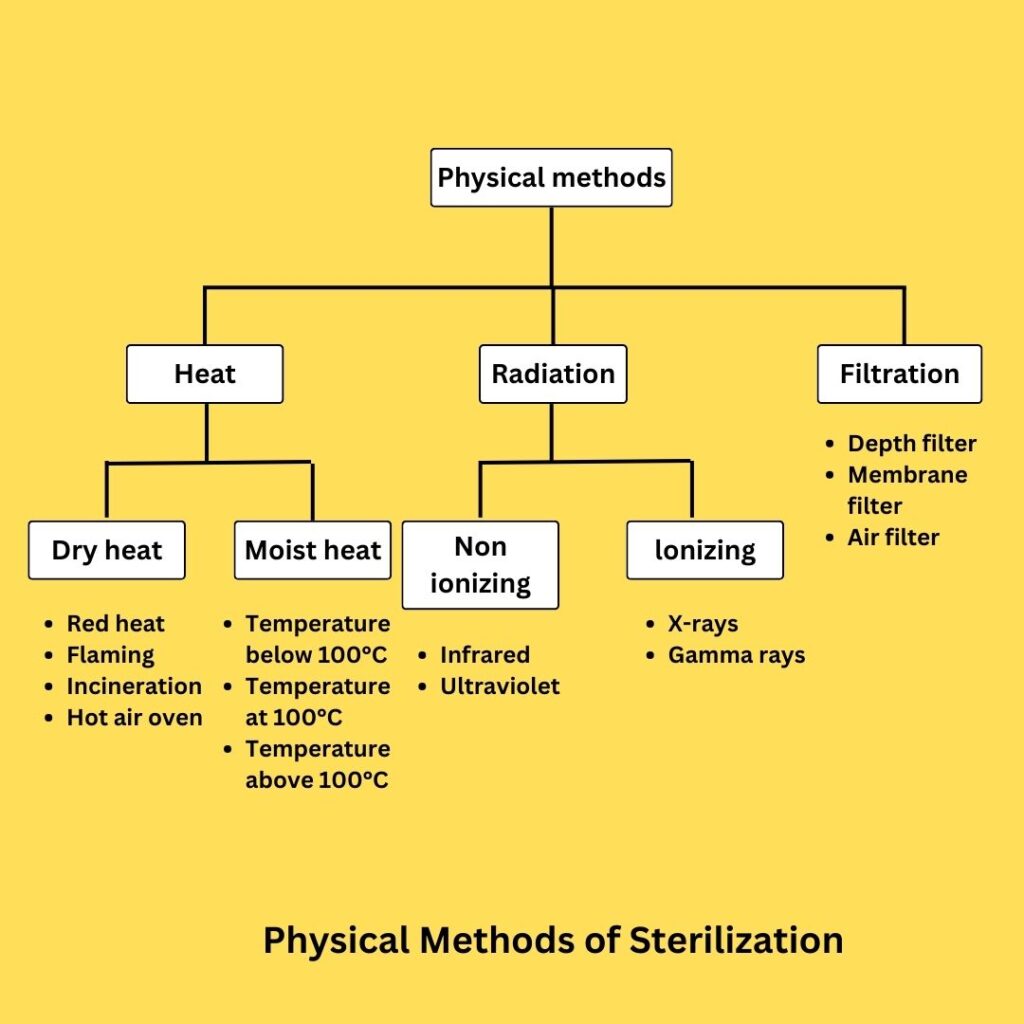
- Sunlight
- Definition: Sunlight, particularly ultraviolet (UV) rays, exhibits a natural germicidal effect that can sterilize water and other surfaces.
- Mechanism: UV rays damage the DNA of microorganisms, leading to their inactivation and subsequent death.
- Application: This method is commonly used for reducing microbial load in natural water sources like lakes and tanks.
- Heat
- Definition: Heat is a prevalent and reliable method of sterilization, utilizing both dry and moist heat to eliminate microorganisms.
- Types and Techniques:
- Dry Heat:
- Principle: Dry heat sterilization works through protein denaturation, oxidative damage, and DNA destruction. It relies on high temperatures to kill microorganisms.
- Procedures:
- Red Heat: Implements the direct flame of a Bunsen burner to sterilize small metal items until they become red hot.
- Flaming: Involves passing items such as glass slides and scalpels through a Bunsen flame without reaching red heat.
- Incineration: Used for the complete destruction of infectious waste by burning it to ashes. This method is applied to soiled dressings, animal carcasses, and other pathological materials.
- Hot Air Oven: Utilizes an electric fan and thermostat to maintain and distribute high temperatures, typically requiring 160°C for two hours, or alternatives such as 170°C for one hour, or 180°C for 30 minutes. It is effective for sterilizing glassware, surgical instruments, and heat-stable chemicals. Control can be monitored using biological indicators like Bacillus subtilis spores.
- Moist Heat:
- Principle: Moist heat sterilization involves denaturation and coagulation of proteins. It is effective at lower temperatures compared to dry heat due to the presence of water, which enhances heat transfer.
- Procedures:
- Pasteurization: Used to reduce pathogenic microbes in milk and other liquids, employing methods such as the Holder method (63°C for 30 minutes) and the flash method (72°C for 20 seconds). The process is effective against most non-sporing pathogens but less so against heat-resistant organisms like Coxiella burnetii.
- Inspissation: Involves heating media at 80-85°C for 30 minutes on three consecutive days using an inspissator. It is used for sterilizing certain culture media.
- Vaccine Bath: Sterilizes bacterial vaccines at 60°C for one hour, and serum or body fluids at 56°C over several days.
- Low-Temperature Steam Formaldehyde Sterilization (LTSF): Utilizes steam at subatmospheric pressure with formaldehyde vapor at 75°C. This method is suitable for materials that cannot withstand higher temperatures, with Bacillus stearothermophilus used as a biological control.
- Boiling: Effective for killing vegetative cells but less so for spores. Typically, boiling for 10-30 minutes is employed when more sophisticated methods are unavailable.
- Tyndallisation: Involves intermittent steaming at 100°C over three days. This method is effective for media that can be damaged by prolonged heat exposure.
- Steam Sterilizer: Uses steam at 100°C for 90 minutes for media that decompose at higher temperatures. It is suitable for less heat-stable materials.
- Autoclave: A high-pressure steam sterilizer that achieves temperatures above 100°C, typically 121.1°C at 15 psi for 15 minutes. It effectively sterilizes culture media, rubber materials, and various medical supplies. The autoclave operates by creating moist conditions that enhance microbial killing, with temperature and pressure monitored through thermocouples and indicators.
- Dry Heat:
- Ozone
- Definition: Ozone sterilization involves generating ozone from oxygen and electricity to provide a non-toxic sterilization method.
- Mechanism: Ozone reacts with microbial cell components to achieve sterilization, with a sterility assurance of 10^-6 in about four hours.
- Temperature Range: Operates between 25-35°C.
- Filtration
- Definition: Filtration removes microorganisms from liquids or gases using various filter types.
- Types:
- Candle Filter: Purifies water by passing it through hollow candles.
- Asbestos Disc Filters: Made from magnesium silicate, used in some laboratory applications.
- Sintered Glass Filters: Prepared by fusing powdered glass, used for various liquid filtrations.
- Membrane Filters: Made from cellulose esters, these are used for water analysis and sterility testing, with pore sizes ranging from 0.015 to 12 microns. The 0.22-micron filter is common for bacteria removal.
- Air Filters: Known as High-Efficiency Particulate Air (HEPA) filters, used in laminar airflow chambers to ensure a bacteria-free environment.
- Limitations: Filters cannot remove viruses due to larger pore sizes.
- Radiation
- Definition: Radiation sterilization involves using ionizing and non-ionizing radiation to inactivate microorganisms.
- Types:
- Ionizing Radiation: Includes gamma rays and X-rays, which penetrate materials to damage microbial DNA. Used commercially for disposable items.
- Non-ionizing Radiation: UV radiation with wavelengths of 240-280 nm causes DNA damage and protein denaturation, used for surface and air sterilization in controlled environments.
| Method | Description | Key Agents/Processes | Applications |
|---|---|---|---|
| Sunlight | Utilizes ultraviolet rays for germicidal effects. Reduces microorganisms in natural bodies of water. | Ultraviolet Rays | Water sterilization |
| Heat | Involves dry heat and moist heat to kill microorganisms through protein denaturation and coagulation. | Dry Heat, Moist Heat | Sterilization of glassware, surgical instruments, chemicals. |
| Dry Heat | Uses high temperatures to induce oxidative damage and protein denaturation. | Red Heat, Flaming, Incineration, Hot Air Oven | Sterilization of metal and glass instruments, chemicals. |
| Moist Heat | Uses steam to kill microorganisms through protein coagulation. | Boiling, Pasteurization, Inspissation, Vaccine Bath, Autoclave | Sterilization of media, vaccines, and heat-sensitive materials. |
| Ozone | Uses ozone gas for disinfection, produced from oxygen through electrical discharges. | Ozone | Sterilization of medical and laboratory equipment. |
| Filtration | Uses filters to remove microorganisms from liquids or gases. | Candle Filters, Asbestos Disc Filters, Sintered Glass Filters, Membrane Filters, Air Filters, Syringe Filters | Sterilization of heat-sensitive liquids and air. |
| Radiation | Utilizes ionizing and non-ionizing radiation to kill microorganisms by damaging DNA. | Gamma Rays, X Rays, UV Radiation | Sterilization of disposable items, surfaces, and environments. |
Uses of Physical Methods of Sterilization
- Sunlight:
- Reducing microbial load in natural water sources such as lakes and tanks.
- Sterilizing surfaces exposed to direct sunlight.
- Heat:
- Dry Heat:
- Red Heat: Sterilizing small metal instruments like inoculation loops.
- Flaming: Sterilizing glass slides and scalpels.
- Incineration: Destroying infectious waste such as soiled dressings and animal carcasses.
- Hot Air Oven: Sterilizing heat-stable glassware, surgical instruments, and chemicals.
- Moist Heat:
- Pasteurization: Sterilizing milk and other liquids.
- Inspissation: Sterilizing culture media.
- Vaccine Bath: Sterilizing bacterial vaccines and body fluids.
- Low-Temperature Steam Formaldehyde Sterilization (LTSF): Sterilizing heat-sensitive materials.
- Boiling: Sterilizing liquids and heat-sensitive items.
- Tyndallisation: Sterilizing media sensitive to prolonged heat.
- Steam Sterilizer: Sterilizing media and materials that decompose at high temperatures.
- Autoclave: Sterilizing culture media, rubber materials, and medical supplies.
- Dry Heat:
- Ozone:
- Sterilizing water and surfaces.
- Providing non-toxic sterilization for various materials.
- Filtration:
- Candle Filter: Purifying water.
- Asbestos Disc Filters: Laboratory applications.
- Sintered Glass Filters: Filtering liquids.
- Membrane Filters: Water analysis, sterility testing, and solution preparation.
- Air Filters (HEPA): Ensuring bacteria-free air in clean rooms and laminar airflow chambers.
- Radiation:
- Ionizing Radiation: Sterilizing disposable items and medical supplies.
- Non-Ionizing Radiation: Sterilizing surfaces and air in controlled environments.
B. Chemical Methods of Sterilization
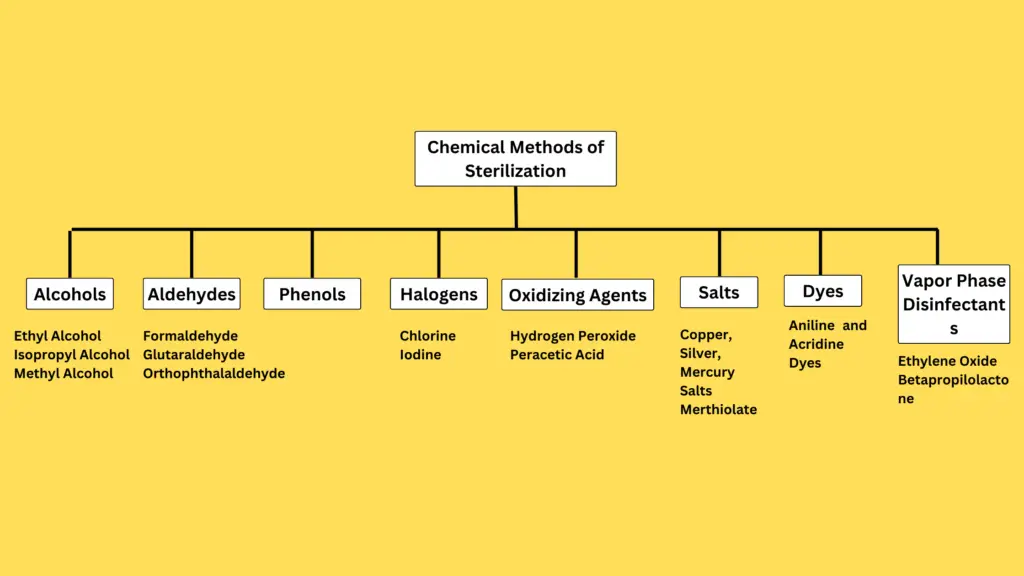
Chemical sterilization utilizes various agents to eliminate microorganisms, including bacteria, viruses, fungi, and protozoa. The effectiveness of chemical disinfectants and antiseptics is determined by several key properties:
- Broad Spectrum of Activity: The chemical agent should target a wide range of microorganisms.
- Penetration Power: Effective agents must penetrate organic matter to ensure thorough disinfection.
- Chemical Stability: The agent should remain stable in both acidic and basic environments.
- Non-Corrosiveness: It should not corrode metals.
- Non-Toxicity: The chemical should be non-toxic if absorbed into circulation.
- Accessibility and Cost: Ideally, the agent should be readily available and economical.
The following outlines the principal chemical methods employed for sterilization.
- Alcohols
- Definition: Alcohols are commonly used disinfectants known for their protein denaturation properties.
- Types and Usage:
- Ethyl Alcohol: Typically used at a concentration of 70% for effective disinfection. It is applied as a skin antiseptic.
- Isopropyl Alcohol: Similar to ethyl alcohol in its mechanism and application, frequently used for skin and surface disinfection.
- Methyl Alcohol: Effective against fungal spores, used for disinfecting inoculation cabinets.
- Aldehydes
- Formaldehyde:
- Definition: A potent disinfectant with bactericidal, sporicidal, and virucidal properties.
- Usage: Employed in both aqueous (10% formalin) and gaseous forms. Used for tissue preservation, vaccine sterilization, and preparation of toxoids.
- Glutaraldehyde:
- Definition: Effective against bacteria, fungi, and viruses, including HIV and hepatitis B. It is known for its less toxic nature.
- Usage: Utilized in a 2% buffered solution for sterilizing medical instruments like cystoscopes and endoscopes.
- Orthophthalaldehyde (OPA):
- Definition: A high-level disinfectant with stability in storage and bactericidal effects against mycobacteria.
- Usage: Used in a 0.5% solution for sterilizing instruments, but precautions are necessary due to respiratory and ocular irritation.
- Formaldehyde:
- Phenols
- Definition: Phenols act as disinfectants by damaging cell membranes, but they can be toxic to skin.
- Derivatives:
- Cresols: Example includes Lysol, used for disinfecting glassware and surfaces.
- Chlorhexidine: Present in solutions like Savlon, effective in wound care and preoperative skin disinfection. It has both bactericidal and fungicidal properties.
- Chloroxylenol: Known as Dettol, it is less toxic and used for general disinfection.
- Hexachlorophene: Acts as a bacteriostatic agent at high dilutions.
- Halogens
- Chlorine:
- Definition: A widely used disinfectant effective in water treatment, swimming pools, and food industries.
- Forms: Includes bleaching powder, sodium hypochlorite, and chloramines. The disinfection action stems from the release of free chlorine, which acts as a strong oxidizing agent.
- Iodine:
- Definition: Utilized in aqueous and alcoholic solutions for skin disinfection. Effective against tuberculosis and has limited activity against spores.
- Forms: Iodophors, iodine compounds combined with surface-active agents, are more effective than iodine solutions alone.
- Chlorine:
- Oxidizing Agents
- Hydrogen Peroxide:
- Definition: Effective against most microorganisms at concentrations of 3-6%, and spores at higher concentrations (10-25%).
- Mechanism: Functions through the liberation of hydroxyl radicals, which are highly reactive and lethal to microorganisms.
- Peracetic Acid:
- Definition: A potent oxidizing agent known for its strong germicidal properties, more effective than hydrogen peroxide.
- Hydrogen Peroxide:
- Salts
- Definition: Salts of heavy metals have disinfectant properties due to their protein coagulating effects.
- Types:
- Copper, Silver, Mercury Salts: These salts act by binding to sulphydryl groups in bacterial proteins, leading to protein coagulation.
- Merthiolate: Sodium ethyl mercurithiosalicylate used in a dilution of 1:10000 for serum preservation.
- Dyes
- Aniline and Acridine Dyes:
- Definition: Both dye groups exhibit bacteriostatic properties.
- Examples:
- Aniline Dyes: Includes crystal violet, brilliant green, and malachite green.
- Acridine Dyes: Includes acriflavine, cuflavin, proflavin, and aminacrine, used for skin and wound antisepsis.
- Aniline and Acridine Dyes:
- Vapor Phase Disinfectants
- Ethylene Oxide (ETO):
- Definition: A colorless liquid used for sterilizing heat-sensitive materials.
- Mechanism: Functions by alkylating amino, carboxyl, hydroxyl, and sulphydryl groups in proteins and nucleic acids.
- Usage: Applied to sterilize plastic and rubber items, medical equipment, and textiles.
- Betapropilolactone (BPO):
- Definition: A condensation product with rapid action used at 0.2% concentration.
- Usage: Efficient in fumigating vaccines and inactivation of biological agents.
- Ethylene Oxide (ETO):
These chemical methods of sterilization are essential in various settings, from medical facilities to laboratory environments, ensuring the effective control of microbial contamination.
Chemical Methods of Sterilization
| Method | Description | Key Agents/Processes | Applications |
|---|---|---|---|
| Alcohols | Denature proteins of microorganisms. | Ethyl Alcohol, Isopropyl Alcohol, Methyl Alcohol | Skin antiseptics, disinfection of surfaces and equipment. |
| Aldehydes | Bactericidal, sporicidal, and virucidal agents. | Formaldehyde, Glutaraldehyde, Orthophthalaldehyde | Sterilization of medical instruments, vaccines, and laboratory equipment. |
| Phenols | Disrupts cell membranes of microorganisms. | Phenol, Cresols, Chlorhexidine, Chloroxylenol, Hexachlorophene | Disinfection of surfaces, wounds, and medical instruments. |
| Halogens | Oxidative agents used to kill microorganisms. | Chlorine, Iodine | Disinfection of water, surfaces, and skin. |
| Oxidizing Agents | Releases radicals or oxygen to kill microorganisms. | Hydrogen Peroxide, Peracetic Acid | Sterilization of surfaces, equipment, and some medical materials. |
| Salts | Uses heavy metal salts to coagulate proteins. | Copper Salts, Silver Salts, Mercury Salts | Disinfection and preservation of sera and other materials. |
| Dyes | Bacteriostatic agents used for skin and wound antiseptics. | Aniline Dyes, Acridine Dyes | Antiseptics for skin and wounds. |
| Vapor Phase Disinfectants | Uses gaseous agents to sterilize materials. | Ethylene Oxide (ETO), Betapropilolactone | Sterilization of heat-sensitive materials and inactivation of vaccines. |
Uses of Chemical Methods of Sterilization
- Alcohols:
- Ethyl Alcohol:
- Applied as a skin antiseptic at a concentration of 70%.
- Used in medical settings for disinfecting hands and surfaces.
- Isopropyl Alcohol:
- Used for skin and surface disinfection.
- Commonly utilized in medical and laboratory environments for cleaning and sanitizing.
- Methyl Alcohol:
- Employed for disinfecting inoculation cabinets.
- Effective against fungal spores in laboratory settings.
- Ethyl Alcohol:
- Aldehydes:
- Formaldehyde:
- Utilized in aqueous (10% formalin) and gaseous forms for tissue preservation.
- Employed for vaccine sterilization and preparation of toxoids.
- Glutaraldehyde:
- Used in a 2% buffered solution for sterilizing medical instruments like cystoscopes and endoscopes.
- Effective against a wide range of microorganisms including HIV and hepatitis B.
- Orthophthalaldehyde (OPA):
- Applied in a 0.5% solution for sterilizing medical instruments.
- Used due to its stability and high-level disinfection properties, with precautions for respiratory and ocular irritation.
- Formaldehyde:
- Phenols:
- Cresols:
- Example: Lysol, used for disinfecting glassware and surfaces.
- Chlorhexidine:
- Found in solutions like Savlon, used for wound care and preoperative skin disinfection.
- Effective against both bacteria and fungi.
- Chloroxylenol:
- Known as Dettol, used for general disinfection due to its lower toxicity.
- Hexachlorophene:
- Acts as a bacteriostatic agent in high dilutions.
- Cresols:
- Halogens:
- Chlorine:
- Used in water treatment, swimming pools, and food industries.
- Applied in forms like bleaching powder, sodium hypochlorite, and chloramines.
- Iodine:
- Utilized in aqueous and alcoholic solutions for skin disinfection.
- Effective against tuberculosis, with iodophors providing enhanced effectiveness.
- Chlorine:
- Oxidizing Agents:
- Hydrogen Peroxide:
- Effective at concentrations of 3-6% for most microorganisms and 10-25% for spores.
- Functions through the release of hydroxyl radicals, which are lethal to microorganisms.
- Peracetic Acid:
- Known for strong germicidal properties, more effective than hydrogen peroxide.
- Used in various sterilization processes due to its potency.
- Hydrogen Peroxide:
- Salts:
- Copper, Silver, Mercury Salts:
- Act by coagulating bacterial proteins through binding to sulphydryl groups.
- Employed in various disinfectant formulations.
- Merthiolate:
- Sodium ethyl mercurithiosalicylate used in a 1:10000 dilution for serum preservation.
- Copper, Silver, Mercury Salts:
- Dyes:
- Aniline Dyes:
- Examples: Crystal violet, brilliant green, malachite green, used for bacteriostatic purposes.
- Acridine Dyes:
- Examples: Acriflavine, cuflavin, proflavin, aminacrine, used for skin and wound antisepsis.
- Aniline Dyes:
- Vapor Phase Disinfectants:
- Ethylene Oxide (ETO):
- A colorless liquid used to sterilize heat-sensitive materials.
- Effective for sterilizing plastic, rubber items, medical equipment, and textiles through alkylation of protein and nucleic acid groups.
- Betapropilolactone (BPO):
- Used at a 0.2% concentration for fumigating vaccines and inactivating biological agents.
- Efficient for rapid action in sterilization processes.
- Ethylene Oxide (ETO):
References
- https://www.brainkart.com/article/Physical-Methods-of-Sterilization_35229/
- https://www.geeksforgeeks.org/sterilization/
- https://www.slideshare.net/slideshow/sterilization-physical-methods/15449594
- https://www.scribd.com/document/600664749/Physical-and-Chemical-Methods-of-Sterilization
- https://medicosage.com/methods-of-sterilization-part-1/
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.