Definition of Simple diffusion
Simple diffusion is one of the types of passive transport that is, as the name implies is the simple movement of solute that happens when the electrochemical potentials of one side of the permeable barrier differ.
- In other disciplines like chemistry, the term diffusion is a term used to describe “spreading out” of molecules that are at a higher concentration in biology. The process is characterized by an eminently permeable membrane in biology.
- Simple diffusion, as with the other mechanisms of passive transport the movement of molecules is based on the gradient of concentration until the concentration of the solute is equal across both sides.
Principle of Simple diffusion/How does simple diffusion work?
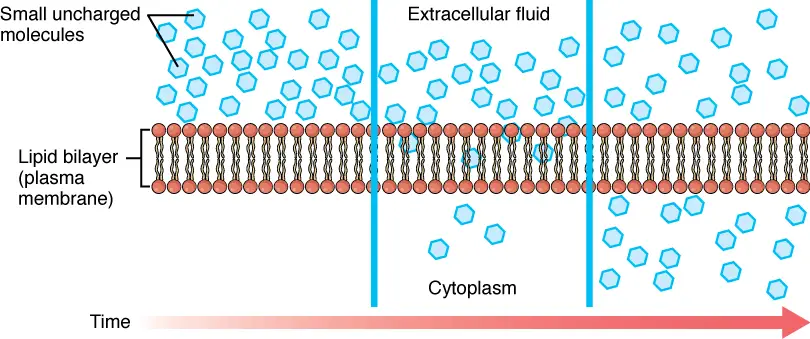
In free diffusion across a membrane, solute particles move around in a randomly Brownian motion, just as they do when in free solution. The solute flux is a tangible and reproducible amount is basically the result of these different motions. While the exact path of a single particle is impossible to predict however, the sum of a huge amount of these pathways is very reproducible.
The flux of free diffusion could be described extremely simply, in the formula that was proposed by Teorell (1953) The formula is: Flux is Mobility + Concentration + Driving Force. It is the amount of moles crossing the membrane in a square centimeter each second. Flux is proportional the solute’s mobility that determines the ease of transportation as well as the concentration which is the measure of the amount of material that is available to participate within the system, and also the driving force.
When the chemical potency of the solute is same across the two components of the membrane, it is in equilibrium so its flux through the membrane will be negligible. When the concentration differs the chemical gradient develops which acts as the primary driver for the flux of the substance. Its mobility solvent is dependent upon the degree of permeability in the membrane that is used for that particular chemical substance. For organisms, the level of permeability needs to be measured by experimentation.
 Principle of Simple diffusion/How does simple diffusion work?” class=”wp-image-15787″/>
Principle of Simple diffusion/How does simple diffusion work?” class=”wp-image-15787″/>Electrolytes’ diffusion
The principle behind charging species’ diffusion dependent on an additional force in conjunction with the gradient of concentration. Solubles that are charged are exposed to electric pressures when electrostatic gradients are in place. Therefore, the primary power for transport of electrolytes is the variation of electrochemical potential, not the chemical potentio. Because any electrolyte solution has to have at the very least one anion and one cation. There are always at least two solvent species that result with multiple fluxes.
Characteristics of Simple diffusion
This is among the most common kinds of passive transport. Other types include diffusion that is facilitated (also called facilitated transportation) filtering, filtration, and Osmosis. Each of them is defined by a downward movement which is the movement of a region that has high concentration to one that is low in concentration. In contrast, active transportation involves an upward transport of substances, i.e. from an area with a lower concentration, to one with a high concentration. The movement happens in the direction of downhill,, or “passive”, chemical energy (ATP) is not required in order for the process to continue.
This is different from different types of membrane transportation in that it is in that they are completely independent. It means that substances don’t make use of membrane proteins to transfer from one place to the next. However Facilitated diffusion requires the channel protein and the carrier protein and osmosis calls for aquaporins (also known as water channels) for molecules to move in and out of cells. Solutes are the molecules which move, in contrast to osmosis, which looks at the movement of solvents (e.g. water) across the plasma membrane. It is easier to understand since it doesn’t rely on the ability of binding membrane proteins.
Factors affecting Simple diffusion
Since the speed of diffusion can be determined using a variety of factors, these parameters affect the process of diffusion.
1. Concentration gradient
- The gradient of concentration across the membrane of a biological cell is the primary force driving the diffusion of a non-electrolyte.
- So, the greater the difference in concentration over the membrane the more is the speed of the diffusion.
- When the spread of molecules on the membrane becomes uniform The rate of diffusion diminishes.
- When equilibrium is established across the membrane, then the diffusion process is stopped.
2. Mass/Size of the solute molecules
- The size of molecules also determines the speed of diffusion across the membrane of a living organism.
- In the event that the dimension of molecules is huge the molecules will find it more difficult to cross the membrane. This will slow speed of dispersion of molecules.
- Therefore this means that diffusion rates are greater for smaller molecules, but slower for larger molecules.
3. Temperature
- Temperature of the systems can also influence the simple diffusion process.
- When temperatures increase the energy of molecules also rises.
- Molecules that have higher energy be more efficient in moving across the membrane , while particles with lower energy are slower.
4. Solubility
- In addition, the solubility properties of particles inside the medium also influences the rate of diffusion of particles.
- Molecules that have lipid-soluble properties can easily move across the lipid layer similar to that of the plasma membrane.
- In the same way, non-polar and polar molecules move at the same speed depending on the properties of the membrane.
5. Solvent density
- With the rise in solvent density diffuse rate slows down.
- A solvent that is more dense will be more difficult for the solvent to travel around.
- Solvent density plays a crucial part in the flow of solute within the cell’s cytoplasm.
- A greater cell’s density can slow the motion of gas and molecules, and it is the reverse for cytoplasm that is less dense.
6. Surface area and thickness of the biological membrane
- Diffusion rates increases as the size of the membrane.
- The increased surface area increases the permeability , or mobility of the molecules . mobility is among the main factors that cause the flux.
- Similar to this it is true that the speed of diffusion also gets decreased by the increase in the thickening of membrane.
Examples of Simple diffusion
1. Oxygen and Carbon dioxide
One of the most famous examples of diffusion that is simple is the flow of gases through the membrane of animals. Carbon dioxide and oxygen dissolving in blood is exchanged through simple diffusion. Based on the gradient in concentration of these gases within the cells and their direction, the gas’s movement is established. In the alveoli, the amount of oxygen is higher when compared to blood vessels when inhaling. Therefore, the flow of oxygen is carried out from the alveoli into blood. Also, during exhalation and exhalation, the level of carbon dioxide increases in blood as opposed to alveoli, which causes the flow of carbon dioxide toward the lung. The same process takes place when gases exchange between blood cells and.
2. Movement of waste materials
Animals, elimination of waste material occurs through simple diffusion. Within the liver waste such as urea is absorbed into blood through the simple process of diffusion. Similar to kidneys elimination of toxins and waste chemicals and the absorption of water happens through simple diffusion. Another active transport occurs in certain areas within the kidneys.
3. Nutrition in bacteria
Prokaryotes such as bacteria don’t have a distinct mechanism for moving nutrients, water, gases and other solutes through their bodies. They depend on simple diffusion to facilitate the circulation of these molecules in the cells. Furthermore, the removal of waste substances in bacteria is also assisted by diffusion that happens through the body’s surface.
Application of Simple diffusion
The idea of diffusion simple is used across a range of areas, such as medical, food and the environment.
- In drinks like soda and tea the dispersion of chemicals and gases out of tea leaves play a crucial element in the formation of the distinctive taste.
- The simple diffusion process is utilized in the action of the body’s medicines. After a medication is taken these molecules release to the specific areas of action via simple diffusion.
- Air pollution is a different phenomenon which is the result of diffusion. The spread of various gases released by industrial, agricultural, and mechanical processes result in air pollution.
- The creation of alloys can also be the result of diffusion. In the long-term exposure of one metal to the other the atoms are able to diffuse from one metal to the next to fill in the gaps. This leads to the formation of various alloys.