What is Schistosoma haematobium?
- Schistosoma haematobium, commonly known as the urinary blood fluke, is a species of digenetic trematode and belongs to the genus Schistosoma. This organism primarily inhabits regions of Africa and the Middle East, where it acts as a significant agent of schistosomiasis, the most widespread parasitic infection affecting humans today. Notably, S. haematobium is distinct among blood flukes for its predilection for the urinary tract, leading to a specific condition known as urinary schistosomiasis.
- The pathological effects of S. haematobium are primarily associated with the eggs produced by the adult flukes, which reside in the venous plexuses surrounding the urinary bladder. Once the eggs are released, they migrate through the bladder wall, resulting in various symptoms such as hematuria, or blood in the urine, as well as bladder fibrosis. Over time, the chronic inflammation caused by these eggs leads to calcification of the bladder, which can exert pressure on the ureters and kidneys, culminating in hydronephrosis.
- A critical concern associated with S. haematobium infection is its potential role in increasing the risk of bladder cancer, ranking only behind tobacco smoking as a contributing factor. Furthermore, the inflammatory responses triggered by this parasite can exacerbate genital inflammation, which may facilitate the transmission of the Human Immunodeficiency Virus (HIV).
- Historically, S. haematobium was the first blood fluke to be identified, with its discovery credited to Theodor Bilharz, a German surgeon, in 1851 while he was working in Cairo. This initial identification led to the broader classification of schistosomiasis as bilharzia or bilharziasis, after its discoverer. In a significant health context, S. haematobium has been classified alongside other helminths, such as Clonorchis sinensis and Opisthorchis viverrini, as a Group 1 carcinogen by the World Health Organization’s International Agency for Research on Cancer (IARC) in 2009, thereby highlighting its serious health implications.
| Kingdom: | Animalia |
| Phylum: | Platyhelminthes |
| Class: | Trematoda |
| Order: | Diplostomida |
| Family: | Schistosomatidae |
| Genus: | Schistosoma |
| Species: | S. haematobium |
History and Distribution of Schistosoma haematobium
- Ancient Evidence: The presence of S. haematobium dates back thousands of years. Notably, eggs from this parasite have been discovered in the renal pelvis of an Egyptian mummy estimated to be from 1,250 to 1,000 BC. Furthermore, schistosome antigens have been identified in mummies from the Predynastic period, around 3,100 BC, using enzyme-linked immunosorbent assay (ELISA) techniques.
- Discovery and Description: The first scientific description of the adult worm occurred in 1851, when Theodor Bilharz identified the parasite while working in Cairo. His findings marked a significant advancement in the understanding of the organism’s role in human health.
- Life Cycle Elucidation: The complete life cycle of S. haematobium, which includes a larval stage in freshwater snails, was elucidated by Leiper in 1915. This discovery was crucial for understanding how the parasite infects hosts and proliferates in endemic areas.
- Geographical Distribution: While S. haematobium is predominantly found in the Nile Valley, it also has a wide distribution across various regions:
- Africa: Endemic in numerous parts of sub-Saharan Africa, where environmental conditions favor the life cycle of the parasite.
- West Asia: Identified in several countries within this region, further expanding its geographical impact.
- India: An isolated focus of endemicity was discovered in Ratnagiri, located south of Mumbai, by researchers Gadgil and Shah in 1952.
- Prevalence and Risk: Globally, approximately 200 million people are considered at risk for infection with S. haematobium, with an estimated 90 million currently infected. This significant prevalence highlights the public health challenge posed by this parasitic infection.
Habitat
Below are the key aspects of the habitat of S. haematobium:
- Adult Worm Location: The adult S. haematobium worms primarily reside in the venous plexuses surrounding the urinary bladder and pelvic region. This specific localization is critical for their reproductive success, as it allows them to release eggs into the urinary tract.
- Life Cycle and Intermediate Hosts:
- Freshwater Snails: The larval stage of S. haematobium develops in freshwater snails, which serve as intermediate hosts. These snails, particularly those from the genus Biomphalaria, are found in various freshwater environments, including rivers, lakes, and irrigation canals.
- Environmental Conditions: The habitat of these snails is essential for the survival of S. haematobium, as they require warm, shallow waters to thrive. Factors such as temperature, pH, and vegetation influence the distribution of these snails and, consequently, the presence of the parasite.
- Geographical Distribution:
- Endemic Regions: While S. haematobium is predominantly found in the Nile Valley, it has a broader geographical distribution, encompassing many areas in sub-Saharan Africa and parts of West Asia. The availability of suitable freshwater habitats in these regions facilitates the life cycle of the parasite.
- Impact of Human Activity: Agricultural practices, urbanization, and water management can significantly alter freshwater habitats, potentially affecting the populations of the intermediate snail hosts. Such changes may lead to increased transmission rates of S. haematobium in human populations.
- Transmission Pathways:
- Human Interaction with Water Sources: The life cycle of S. haematobium is closely associated with human activities, particularly in areas where people frequently come into contact with freshwater bodies. This includes activities such as bathing, washing, and fishing, which increase the likelihood of exposure to the infective larval forms, known as cercariae.
Hosts of Schistosoma haematobium
Below are the key aspects regarding its hosts:
- Definitive Hosts:
- Humans: The primary definitive host for S. haematobium is humans. The adult worms reside in the venous plexuses of the urinary bladder and pelvic region, where they can reproduce and release eggs.
- Zoonotic Potential: Unlike some other schistosome species, S. haematobium is predominantly a human parasite. It has not been widely documented in animal hosts, which differentiates it from species such as S. japonicum and S. mansoni, which have significant animal reservoirs.
- Intermediate Hosts:
- Freshwater Snails: The lifecycle of S. haematobium is dependent on specific freshwater snails that act as intermediate hosts. The primary genus associated with S. haematobium is Bulinus.
- Bulinus truncatus: This species is particularly important, as it facilitates the development of the larval forms of S. haematobium, allowing the parasite to infect humans when cercariae are released into the water.
- Environmental Conditions for Snails: The presence and abundance of these snails in freshwater bodies are influenced by environmental factors such as temperature, vegetation, and water quality, which in turn affect the transmission of S. haematobium.
- Freshwater Snails: The lifecycle of S. haematobium is dependent on specific freshwater snails that act as intermediate hosts. The primary genus associated with S. haematobium is Bulinus.
- Other Related Species:
- Other schistosome species utilize different intermediate hosts. For example:
- S. mansoni: Uses snails from the genus Biomphalaria as its intermediate hosts.
- S. japonicum: Relies on Oncomelania snails.
- S. mekongi: Primarily depends on the snail Neotricula aperta for its lifecycle.
- Other schistosome species utilize different intermediate hosts. For example:
Characteristics of Schistosoma haematobium
- Size and shape: Mature Schistosoma haematobium worms are between 6 and 20 mm in length and have a flattened, elongated form.
- Lifecycle: The complex lifecycle of Schistosoma haematobium involves an intermediate host (a freshwater snail) and a definitive host (humans). Adult worms reside in the veins that drain the bladder, where they lay eggs that are expelled by urine.
- Transmission: Schistosoma haematobium is transmitted to humans when they come into touch with contaminated waters that contains the intermediate host snail. The snail’s larvae enter the epidermis of the human host and move to the bladder veins, where they mature into adult worms.
- Symptoms: Symptoms associated with Schistosoma haematobium infection include bloody urine, stomach pain, and diarrhoea. Chronic infections can cause damage to the bladder and kidneys.
- Treatment: Praziquantel, an antiparasitic medicine that kills adult worms, is the most common treatment for Schistosoma haematobium infection. Access to clean water and good sanitation are also effective in preventing the spread of the disease.
Morphology of Schistosoma haematobium
Below are the key morphological characteristics of S. haematobium, encompassing both the adult worm and its eggs.
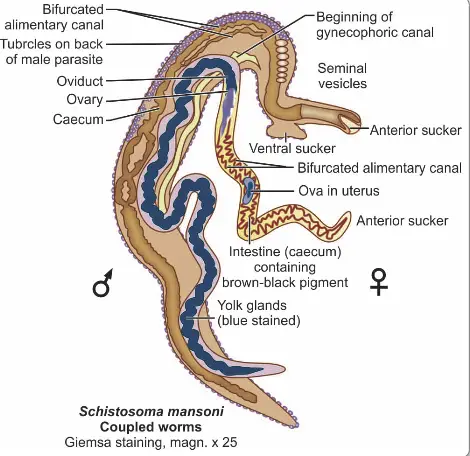
- Adult Worm Structure:
- Sexual Dimorphism: S. haematobium exhibits notable sexual dimorphism, with the male and female worms differing significantly in size and shape.
- Male Morphology:
- Length: Approximately 10–15 mm and a thickness of about 1 mm.
- Cuticle: The male is covered with a finely tuberculated cuticle, providing protection and aiding in its attachment to the host’s tissues.
- Suckers: It possesses two muscular suckers, including a small oral sucker and a prominent ventral sucker. These structures facilitate feeding and anchorage within the host.
- Gynecophoric Canal: A unique feature of the male is the gynecophoric canal, which runs from the ventral sucker to the caudal end of the body. This canal serves to hold the female worm during copulation.
- Female Morphology:
- Length: The adult female is longer and more slender than the male, measuring approximately 20 mm in length and 0.25 mm in width.
- Cuticular Features: The tubercles are confined to the anterior and posterior ends, contrasting with the male’s more uniformly distributed tubercles.
- Reproductive Capacity: The gravid female can contain 20–30 eggs in her uterus simultaneously and may expel up to 300 eggs daily.
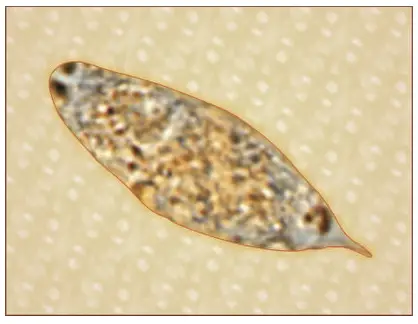
- Egg Structure:
- Size and Shape: The eggs of S. haematobium are ovoid, measuring about 150 μm by 50 μm. Their shape is crucial for their passage through host tissues.
- Shell Characteristics: The eggs possess a non-operculated, brownish-yellow transparent shell that carries a distinctive terminal spine at one pole. This spine plays a vital role in the egg’s expulsion mechanism.
- Mechanism of Egg Expulsion:
- Site of Egg Laying: The eggs are typically laid in the small venules of the vesical and pelvic plexuses. Occasionally, they may also be found in ectopic sites such as the mesenteric portal system and pulmonary arterioles.
- Egg Arrangement: Eggs are laid sequentially, with the terminal spine oriented posteriorly. This arrangement facilitates movement through the host tissues.
- Penetration Mechanism: The eggs utilize the terminal spine to penetrate the vesical wall, aided by the pressure within the venules and a lytic substance released by the eggs. This mechanism allows the eggs to enter the lumen of the urinary bladder.
- Discharge in Urine: The eggs are expelled during micturition, often more frequently observed during midday, although the exact reasons for this timing remain unclear.
- Ectopic Egg Fate: Eggs laid in ectopic sites generally do not survive and may provoke localized tissue reactions, although they are rarely expelled live in feces.
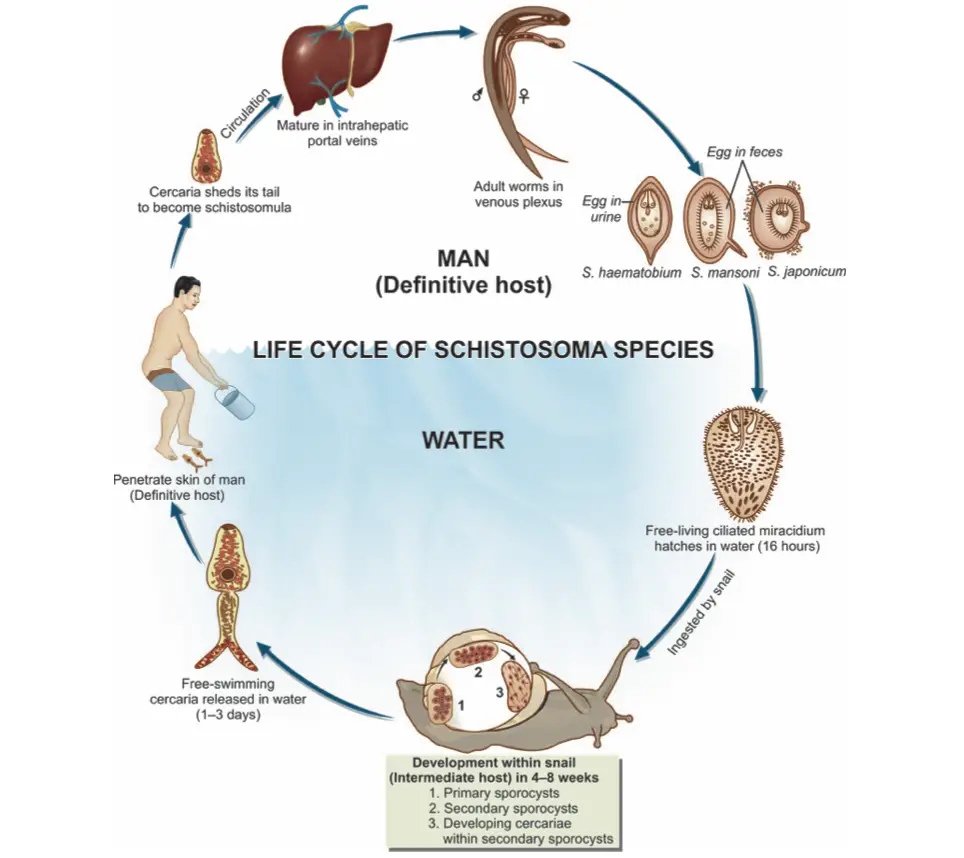
Life cycle of Schistosoma haematobium
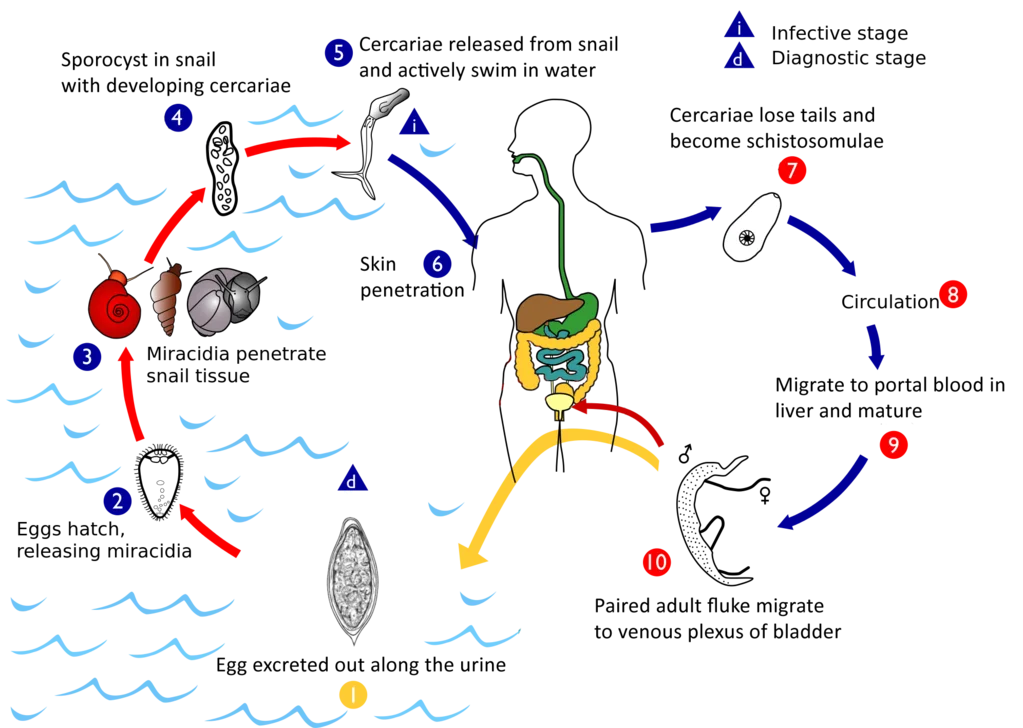
The following points outline the key stages and processes involved in the life cycle of S. haematobium.
- Egg Elimination:
- Eggs are excreted from the human host either through feces or urine, depending on the specific species of schistosome. The elimination method is critical for the continuation of the life cycle.
- Hatching of Eggs:
- Under suitable environmental conditions, the eggs hatch and release free-swimming miracidia. These larvae are motile, equipped with cilia that allow them to navigate through water.
- Penetration of Snail Hosts:
- Miracidia are attracted to specific snail species, primarily belonging to the Bulinus genus in Africa, and the limpet Ferrisia tenuis in India. Upon contact, they penetrate the snail’s tissue and migrate to the liver.
- Development in Snail:
- Inside the snail, the miracidia lose their cilia and undergo several transformations. This process includes:
- Formation of two generations of sporocysts, which can be described as asexual reproductive structures.
- The production of a large number of cercariae through asexual reproduction within the second-generation sporocysts.
- Inside the snail, the miracidia lose their cilia and undergo several transformations. This process includes:
- Release of Cercariae:
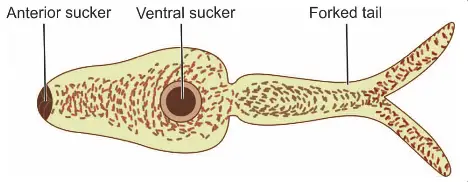
- After maturation, the cercariae are released into the aquatic environment. These cercariae possess an elongated ovoid body with a forked tail, which aids in swimming.
- Human Infection:
- The infective cercariae swim actively for a short duration, typically 1 to 3 days. If they come into contact with a human host, they penetrate the skin. This penetration is facilitated by lytic substances secreted from specialized glands in the cercariae.
- Transformation to Schistosomulae:
- Upon entering the human host, cercariae shed their tails and transform into schistosomulae. These schistosomulae then enter the venous circulation.
- Migration Through the Body:
- Schistosomulae migrate through the venous system, passing through the lungs and then into the heart. Subsequently, they reach the liver, where they undergo maturation.
- Maturation and Reproduction:
- After approximately 20 days, schistosomulae mature into sexually differentiated adult worms in the intrahepatic portal veins. Male and female worms copulate and typically reside in specific locations within the mesenteric venous system.
- S. haematobium predominantly inhabits the vesicular and pelvic venous plexuses of the bladder, but it can also be found in the rectal venules.
- After approximately 20 days, schistosomulae mature into sexually differentiated adult worms in the intrahepatic portal veins. Male and female worms copulate and typically reside in specific locations within the mesenteric venous system.
- Egg Production:
- Female worms, ranging in size from 7 to 28 mm, deposit their eggs in small venules of the portal and perivesical systems. The deposition site varies among species:
- S. mansoni and S. japonicum primarily target the intestinal tract.
- S. haematobium focuses on the urinary tract, with eggs moving progressively towards the bladder and ureters.
- Female worms, ranging in size from 7 to 28 mm, deposit their eggs in small venules of the portal and perivesical systems. The deposition site varies among species:
- Excretion of Eggs:
- The eggs eventually reach the lumen of the intestine (for S. mansoni, S. japonicum, etc.) or the bladder and ureters (S. haematobium). They are then excreted with feces or urine, thus completing the life cycle.
Pathogenicity and Clinical Features of Schistosoma haematobium
The pathogenicity and clinical features of Schistosoma haematobium, a major contributor to human schistosomiasis, are critical for understanding the disease’s progression and its impact on health. This trematode’s life cycle and the host’s immune response play significant roles in the manifestation of various clinical symptoms. The clinical illness resulting from S. haematobium infection can be categorized into three distinct phases, reflecting the stages of infection evolution: skin penetration and incubation period, egg deposition and extrusion, and tissue proliferation and repair.
- Skin Penetration and Incubation Period:
- The initial phase of infection begins when cercariae penetrate the skin, leading to potential local reactions. During this incubation period, the following symptoms may arise:
- Cercarial Dermatitis: Characterized by transient itching and petechial lesions at the site of entry, this condition, also known as swimmer’s itch, is more prevalent among visitors to endemic regions than among local populations. Locals often develop immunity due to repeated exposure.
- Anaphylactic or Toxic Symptoms: These can include systemic reactions such as fever, headache, malaise, and urticaria. Accompanying laboratory findings typically include leukocytosis and eosinophilia, along with signs of liver enlargement (hepatomegaly) and splenomegaly. This phase is notably associated with S. japonicum infections, which may lead to a condition known as Katayama fever.
- The initial phase of infection begins when cercariae penetrate the skin, leading to potential local reactions. During this incubation period, the following symptoms may arise:
- Egg Deposition and Extrusion:
- Once the adult worms have matured, the clinical features during this phase are predominantly urinary in nature, characterized by:
- Painless Terminal Hematuria: This symptom, also referred to as endemic hematuria, typically begins as microscopic hematuria, which may progress to gross hematuria in cases of heavy infection.
- Frequency of Micturition and Dysuria: Most patients experience increased urinary frequency and burning sensations.
- Cystoscopic Findings: Cystoscopy often reveals hyperplasia and inflammation of the bladder mucosa, presenting with minute papular or vesicular lesions.
- Once the adult worms have matured, the clinical features during this phase are predominantly urinary in nature, characterized by:
- Tissue Proliferation and Repair:
- In the chronic stage of infection, the following pathological changes occur:
- Hyperplasia and Fibrosis: The vesical mucosa may exhibit generalized hyperplasia and fibrosis, leading to a granular appearance often referred to as sandy patches.
- Inflammatory Response: At egg deposition sites, there is significant infiltration of lymphocytes, plasma cells, and eosinophils, forming pseudoabscesses. Initially, this inflammation tends to affect the trigone area but can extend to involve the entire mucosa, resulting in thickening and ulceration.
- Secondary Infections: Chronic cystitis may develop due to secondary bacterial infections.
- Formation of Calculi: The deposition of oxalate and uric acid crystals around eggs and blood clots may lead to the formation of bladder stones, which can result in obstructive hyperplasia of the ureters and urethra (hydroureter).
- Carcinogenic Potential: Chronic schistosomiasis has been linked to an increased risk of squamous cell carcinoma of the bladder, often diagnosed at a younger age than transitional cell carcinoma. As such, S. haematobium is classified as a human carcinogen.
- In the chronic stage of infection, the following pathological changes occur:
- Ectopic Manifestations:
- Besides urinary symptoms, S. haematobium can also impact other organ systems, leading to significant morbidity:
- Involvement of the lungs, central nervous system (e.g., transverse myelitis-like syndromes), skin, and genital organs can occur, with ectopic lesions posing additional health risks.
- Moreover, schistosomiasis may promote urinary carriage of typhoid bacilli, complicating the clinical picture.
- Besides urinary symptoms, S. haematobium can also impact other organ systems, leading to significant morbidity:
Laboratory Diagnosis of Schistosoma haematobium
The laboratory diagnosis of Schistosoma haematobium is critical for effective management and treatment of schistosomiasis. Accurate identification of the parasite involves various techniques, each targeting different aspects of the infection. The following methods are commonly employed in the diagnostic process:
- Urine Microscopy:
- Microscopic examination of urine is the primary method for detecting S. haematobium eggs. The key features include:
- Centrifuged Deposits: A centrifugation method is used to concentrate the urine samples, allowing for easier visualization of the eggs.
- Filtration Technique: Alternatively, a known volume of urine can be filtered through nucleopore filters. This technique not only reveals the presence of eggs but also quantifies the intensity of infection.
- Distribution of Eggs: It is important to note that eggs are more prevalent in the blood and pus expelled at the end of micturition, which is essential for accurate sampling.
- Presence in Seminal Fluid: In males, S. haematobium eggs can also be detected in seminal fluid, providing another potential source for diagnosis.
- Microscopic examination of urine is the primary method for detecting S. haematobium eggs. The key features include:
- Histopathology:
- Biopsy of the bladder mucosa can confirm schistosome infection by revealing the presence of eggs. This invasive method is useful in cases where other diagnostic techniques yield inconclusive results.
- Detection of Antigens:
- The identification of specific schistosome antigens in serum or urine has emerged as a valuable diagnostic method. Key points include:
- Glycoprotein Antigens: Two significant glycoprotein antigens are the circulating anodic antigen (CAA) and circulating cathodic antigens (CCA).
- ELISA Utilization: These antigens can be detected using enzyme-linked immunosorbent assays (ELISA) employing monoclonal antibodies. This method is highly sensitive and specific, but it is generally restricted to specialized laboratories.
- The identification of specific schistosome antigens in serum or urine has emerged as a valuable diagnostic method. Key points include:
- Detection of Antibodies:
- Various serological tests have been developed to identify specific antibodies, though their utility is limited. Important considerations include:
- Limited Specificity: Many serological tests cannot distinguish between current and past infections.
- Types of Tests: Techniques such as complement fixation test (CFT), bentonite flocculation test, indirect hemagglutination (IHA), immunofluorescence, and gel diffusion tests have been employed, but are less common.
- CDC-Approved Tests: The falcon assay screening test (FAST)/ELISA and enzyme-linked immunoelectrotransfer blot (EITB) are highly sensitive and specific tests available through the Centers for Disease Control and Prevention (CDC). In particular, the FAST ELISA uses S. haematobium adult worm microsomal antigen (HAMA) for antibody detection.
- Various serological tests have been developed to identify specific antibodies, though their utility is limited. Important considerations include:
- Intradermal Skin Test (Fairley’s Test):
- This group-specific skin test can yield positive results across all forms of schistosomiasis. The intradermal allergic response employs antigens derived from infected snails, cercariae, eggs, and adult schistosomes from experimentally infected animals.
- Imaging Techniques:
- Imaging studies provide complementary information for diagnosing S. haematobium infections. Notable techniques include:
- X-ray Imaging: Abdominal X-rays may reveal calcifications in the bladder and ureters, indicative of chronic infection.
- Ultrasonography (USG): This non-invasive technique is effective in visualizing complications such as hydroureter and hydronephrosis, which may arise from the obstruction caused by schistosomiasis.
- Intravenous Pyelogram (IVP) and Cystoscopy: These procedures assist in the indirect diagnosis of the disease, providing valuable insights into the urinary tract’s condition.
- Imaging studies provide complementary information for diagnosing S. haematobium infections. Notable techniques include:
Treatment of Schistosoma haematobium
The treatment of Schistosoma haematobium infection primarily involves the use of specific anthelmintic medications. Effective treatment aims to eliminate the parasite, alleviate symptoms, and prevent potential complications. The following outlines the main treatment options available for this condition:
- Praziquantel:
- Drug of Choice: Praziquantel is the first-line medication for treating S. haematobium infections.
- Dosage: The standard dosage is 40 mg/kg, administered as a single day-long treatment.
- Mechanism of Action: Praziquantel works by inducing severe spasms and paralysis of the worms, which are then dislodged from their attachment sites in the host’s tissues, facilitating their elimination through the host’s immune system and excretory pathways.
- Efficacy: This medication is well-tolerated and demonstrates high efficacy against the adult forms of the parasite, significantly reducing egg output and associated morbidity.
- Metriphonate:
- Alternative Drug: Metriphonate serves as a secondary treatment option, particularly when Praziquantel is unavailable or contraindicated.
- Dosage: The recommended dosage is 7.5 mg/kg, administered weekly for three consecutive weeks.
- Mechanism of Action: Metriphonate acts by inhibiting the enzyme acetylcholinesterase in the parasite, which leads to neuromuscular paralysis, thus facilitating the clearance of the infection.
- Use in Practice: While it is effective, Metriphonate is less commonly used than Praziquantel, mainly due to its dosing schedule and potential side effects.
- Monitoring and Follow-Up:
- Post-Treatment Assessment: Following treatment, it is essential to monitor patients for clinical improvement and potential recurrence of infection. This can involve follow-up urinalysis to check for residual eggs.
- Management of Complications: In cases where chronic schistosomiasis has led to significant tissue damage or complications, additional medical interventions may be necessary. This could include managing urinary tract obstructions or addressing secondary infections.
- Public Health Considerations:
- Preventive Measures: In endemic regions, controlling the snail intermediate hosts and improving sanitation practices are crucial to reducing transmission. Education about avoiding contact with contaminated water sources is vital for prevention.
- Community Treatment Programs: Mass drug administration strategies can be effective in reducing the prevalence of S. haematobium infection within communities at risk.
Prophylaxis of Schistosoma haematobium
Prophylaxis of Schistosoma haematobium infection is crucial for reducing transmission and preventing the associated morbidity. Effective strategies can be implemented at both the community and individual levels. The following outlines the primary prophylactic measures:
- Eradication of Intermediate Hosts:
- Targeting Snail Populations: The primary intermediate hosts of S. haematobium are specific species of freshwater snails. Therefore, controlling and reducing these snail populations is essential.
- Methods of Control: This can be achieved through the application of molluscicides, habitat modification, and improving water management practices to reduce breeding sites for snails.
- Prevention of Environmental Pollution:
- Sanitation Measures: It is vital to implement effective sanitation practices to prevent the contamination of water bodies with urine and feces. Poor sanitation can facilitate the life cycle of schistosomes and increase the risk of transmission.
- Waste Management: Ensuring proper disposal of human waste and promoting the use of latrines can significantly diminish environmental contamination.
- Effective Treatment of Infected Individuals:
- Screening and Treatment Programs: Identifying and treating individuals infected with S. haematobium not only benefits the affected individuals but also reduces the overall parasite load in the community, lowering the risk of further transmission.
- Mass Drug Administration: In endemic regions, implementing community-wide treatment initiatives can help prevent outbreaks and reduce the prevalence of infection.
- Avoidance of Infected Water Bodies:
- Personal Preventive Measures: Individuals should be advised to avoid swimming, bathing, or washing in freshwater sources known to be contaminated with S. haematobium cercariae. This is especially important for those living in or visiting endemic areas.
- Public Awareness Campaigns: Educational initiatives should be established to raise awareness about the risks associated with contact with potentially infected water and the importance of preventive behaviours.
- Community Engagement:
- Local Involvement: Encouraging community involvement in prevention strategies can lead to more sustainable outcomes. This includes educating locals about the life cycle of the parasite and promoting community-led sanitation initiatives.
- Monitoring and Surveillance:
- Regular Surveillance: Continuous monitoring of water bodies and snail populations is necessary to identify potential risks early. This can facilitate timely interventions to prevent outbreaks.
- Health Surveillance: Tracking infection rates within the community enables health authorities to tailor interventions effectively and allocate resources where they are most needed.
Facts about Schistosoma haematobium
- The parasitic flatworm Schistosoma haematobium causes the diseases schistosomiasis and bilharzia.
- It is one of the five human-infecting Schistosoma species.
- Schistosoma haematobium is present across Africa, the Middle East, and the islands of the Indian Ocean.
- Larvae of the parasite are discharged into the water by infected freshwater snails, where they can penetrate human skin upon contact with infected water.
- The parasite then grows in the veins surrounding the bladder and excretes eggs through the urine.
- The transmission of Schistosoma haematobium infection occurs by contact with contaminated water.
- The disease is especially prevalent in rural, underprivileged communities with inadequate access to potable water and sanitation.
- In many regions of Africa, Schistosoma haematobium infection is considered to be endemic.
- Schistosoma haematobium affects an estimated 112 million people around the world.
- Blood in the urine, painful urination, abdominal pain, diarrhoea, and exhaustion may be symptoms of a Schistosoma haematobium infection.
- The degree of symptoms may vary according on the duration and severity of an infection.
- Schistosoma haematobium infection is diagnosed by finding parasite eggs in a urine sample or a bladder or ureter biopsy.
- Praziquantel is the most common medicine used to treat Schistosoma haematobium infection.
- Generally, treatment is well tolerated and side effects are minor.
- If left untreated, a Schistosoma haematobium infection can cause persistent health issues such as bladder and kidney damage.
- Persistent infections can cause renal failure, and in extreme circumstances, they can be fatal.
- The transmission of Schistosoma haematobium can be avoided by avoiding contact with polluted water.
- Swimming or wading in freshwater while wearing protective equipment can also lower the risk of infection.
- Schistosoma haematobium infection can also be avoided by providing safe drinking water and proper sanitation.
- Treatment of sick persons is also essential for avoiding the infection’s spread.
FAQ
What is Schistosoma haematobium?
Schistosoma haematobium is a parasitic flatworm that causes a disease called schistosomiasis or bilharzia. It is one of the five species of Schistosoma that infect humans.
How is Schistosoma haematobium transmitted?
Schistosoma haematobium is transmitted through contact with contaminated water. The parasite’s larvae are released from infected freshwater snails into the water, where they can penetrate human skin during contact with infected water. The parasite then matures in the veins around the bladder and releases eggs, which are excreted in the urine.
What are the symptoms of Schistosoma haematobium infection?
The symptoms of Schistosoma haematobium infection can vary depending on the duration and intensity of the infection. Symptoms may include blood in the urine, painful urination, abdominal pain, diarrhea, and fatigue.
How is Schistosoma haematobium diagnosed?
Diagnosis of Schistosoma haematobium infection is made by identifying the parasite’s eggs in a urine sample or a biopsy of the bladder or ureter.
How is Schistosoma haematobium treated?
The most commonly used drug for treating Schistosoma haematobium infection is praziquantel. This medication is usually given as a single dose. Treatment is generally well-tolerated, and side effects are usually mild.
How can Schistosoma haematobium infection be prevented?
Schistosoma haematobium infection can be prevented by avoiding contact with contaminated water, wearing protective clothing when swimming or wading in freshwater, and practicing good hygiene. It is also important to treat infected individuals and to provide safe drinking water and adequate sanitation.
Where is Schistosoma haematobium found?
Schistosoma haematobium is found in parts of Africa, the Middle East, and the Indian Ocean islands.
How common is Schistosoma haematobium infection?
Schistosoma haematobium infection is considered to be endemic in many parts of Africa, with an estimated 112 million people infected worldwide. The infection is most common in rural and impoverished communities with poor access to safe drinking water and sanitation.
What is the long-term outlook for someone with Schistosoma haematobium infection?
If left untreated, Schistosoma haematobium infection can lead to chronic health problems such as bladder and kidney damage, which can result in renal failure. In severe cases, the infection can be fatal. However, with proper treatment, the prognosis is generally good.
- Nelwan ML. Schistosomiasis: Life Cycle, Diagnosis, and Control. Curr Ther Res Clin Exp. 2019 Jun 22;91:5-9. doi: 10.1016/j.curtheres.2019.06.001. PMID: 31372189; PMCID: PMC6658823.
- Nelwan, M. L. (2019). Schistosomiasis: Life Cycle, Diagnosis, and Control. Current Therapeutic Research, 91, 5–9. doi:10.1016/j.curtheres.2019.06.0
- Rose, Matthew & Zimmerman, Eli & Hsu, Liangge & Golby, Alexandra & Saleh, Emam & Folkerth, Rebecca & Santagata, Sandro & Milner, Danny & Ramkissoon, Shakti. (2014). Atypical presentation of cerebral schistosomiasis four years after exposure to Schistosoma mansoni. Epilepsy & Behavior Case Reports. 2. 10.1016/j.ebcr.2014.01.006.
- https://www.cartercenter.org/resources/pdfs/health/schistosomiasis/schisto-disease-cycle.pdf
- https://www.cdc.gov/dpdx/schistosomiasis/index.html
- https://animaldiversity.org/accounts/Schistosoma_haematobium/
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.