SARS-CoV-2 is the virus that causes COVID-19. COVID-19 is a highly infectious respiratory illness that can spread from person to person through respiratory droplets or close contact. The virus was first identified in Wuhan, China in December 2019 and has since spread to become a global pandemic. Symptoms of COVID-19 can range from mild to severe and may include fever, cough, and difficulty breathing. The virus can be severe or even deadly, particularly for older adults or people with underlying health conditions. Governments and health agencies around the world have implemented measures such as lockdowns, travel restrictions, and mask-wearing to slow the spread of the virus.
Modes of transmission of SARS-CoV-2 (COVID-19)
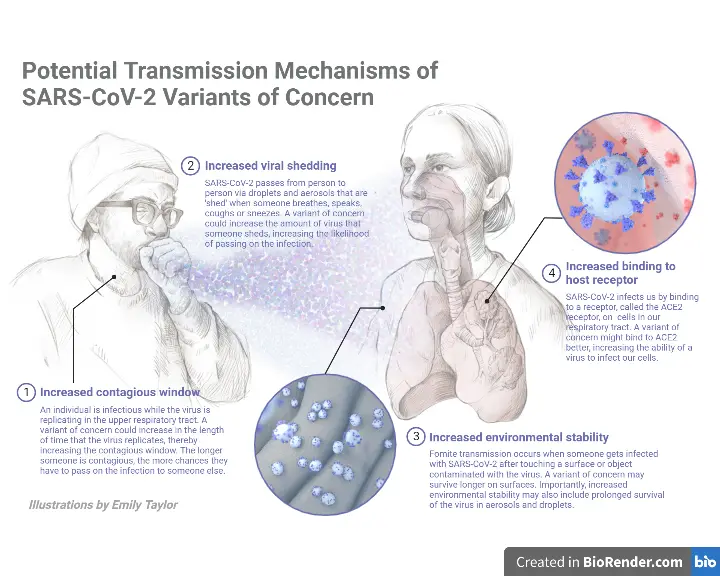
This section explains briefly the several ways of SARS-CoV-2 transmission, including contact, droplet, airborne, fomite, fecal-oral, bloodborne, mother-to-child, and animal-to-human transmission. Some patients infected with SARS-CoV-2 never develop symptoms. SARS-CoV-2 infection primarily causes respiratory sickness ranging from moderate to severe disease and death.
Contact and droplet transmission
- SARS-CoV-2 can be transmitted through direct, indirect, or near contact with an infected person’s saliva, respiratory secretions, or respiratory droplets, which are expelled when an infected person coughs, sneezes, talks, or sings.
- Respiratory droplets have a diameter of 5-10 m or greater, whereas droplets with a diameter of 5m are known as droplet nuclei or aerosols.
- Respiratory droplet transmission can occur when a person is in close contact (within 1 metre) with an infected person who has respiratory symptoms (such as coughing or sneezing) or who is talking or singing; under these conditions, virus-containing respiratory droplets can reach the mouth, nose, or eyes of a susceptible person and cause infection.
- Also feasible is indirect contact transmission between a vulnerable host and a contaminated object or surface (fomite transfer).
Airborne transmission
- Airborne transmission is the propagation of an infectious agent induced by the dispersal of droplet nuclei (aerosols) that remain infectious when floating in the air across large distances and periods of time.
- SARS-CoV-2 can be transmitted through the air during medical procedures that produce aerosols (“aerosol-generating operations”).
- WHO and the scientific community have been actively discussing and reviewing whether SARS-CoV-2 can transmit by aerosols in the absence of aerosol-generating techniques, especially in indoor environments with poor ventilation.
- Possible methods for SARS-CoV-2 transmission through aerosols have been hypothesised based on the physics of exhaled air and flow physics.
- These hypotheses propose that 1) a number of respiratory droplets form microscopic aerosols (5 m) by evaporation, and 2) regular breathing and speech generate exhaled aerosols. Therefore, a susceptible individual could inhale aerosols and become infected if the aerosols contain enough virus to trigger infection in the recipient.
- However, the fraction of exhaled droplet nuclei or respiratory droplets that evaporate to produce aerosols, as well as the infectious dose of viable SARS-CoV-2 required to infect another individual, remain unknown; however, these variables have been investigated for other respiratory viruses.
Fomite transmission
- Infected persons’ respiratory secretions or droplets can contaminate surfaces and items, causing fomites (contaminated surfaces).
- Viable SARS-CoV-2 virus and/or RNA detected by RT-PCR can be found on surfaces for periods ranging from hours to days, depending on the ambient environment (including temperature and humidity) and the type of surface, especially in high concentrations in health care facilities where COVID-19 patients were treated.
- Touching surfaces in the local area or things contaminated with virus from an infected individual (such as a stethoscope or thermometer) and then touching the mouth, nose, or eyes can therefore also result in transmission.
- Despite continuous evidence of SARS-CoV-2 contamination of surfaces and the virus’s persistence on some surfaces, there are no studies that establish fomite transmission directly.
- People who come into contact with potentially infectious surfaces frequently have close contact with the infectious individual, making it difficult to distinguish between respiratory droplet and fomite transmission.
- Nevertheless, fomite transmission is thought to be a likely mode of SARS-CoV-2 transmission, given persistent observations of environmental contamination in the neighbourhood of infected individuals and the fact that other coronaviruses and respiratory viruses can spread in this manner.
Other modes of transmission
- SARS-CoV-2 RNA has also been identified in the urine and faeces of certain patients. In one investigation, live SARS-CoV-2 was detected in the urine of one patient. SARS-CoV-2 has been cultivated three times from stool samples.
- To date, there have been no documented reports of SARS-CoV-2 transmission via faeces or urine.
- Some investigations have detected SARS-CoV-2 RNA in plasma or serum, and the virus is capable of replicating within blood cells.
- However, the function of bloodborne transmission is yet unknown, and low virus titers in plasma and serum indicate that the risk of transmission by this route is likely minimal.
- There is no indication of intrauterine transmission of SARS-CoV-2 from infected pregnant women to their foetuses at this time, however data remain restricted. The World Health Organization recently published a scientific summary on breastfeeding and COVID-19.
- This summary indicates that RT-PCR testing has detected viral RNA fragments in a few breast milk samples from moms infected with SARS-CoV-2, but investigations assessing the viability of the virus have found none. In order for SARS-CoV-2 to be transmitted from mother to child, replicative and infectious virus must be able to reach target locations in the infant and also defeat infant defensive systems. WHO suggests encouraging moms with suspected or confirmed COVID-19 to commence or continue breastfeeding.
- Currently available evidence indicates that SARS-CoV-2 is most closely linked to known betacoronaviruses in bats; nevertheless, the function of an intermediate host in enabling transmission in the earliest known human cases remains unclear.
- In addition to investigations on the potential intermediate host(s) of SARS-CoV-2, a number of studies are currently underway to better understand SARS-CoV-2 susceptibility in various animal species. Current research suggests that SARS-CoV-2 infected humans can transmit the virus to other species, including dogs, cats, and domesticated mink. However, it is unknown if these diseased mammals constitute a major danger of human infection.
Pathogenesis of SARS-CoV-2 (COVID-19)
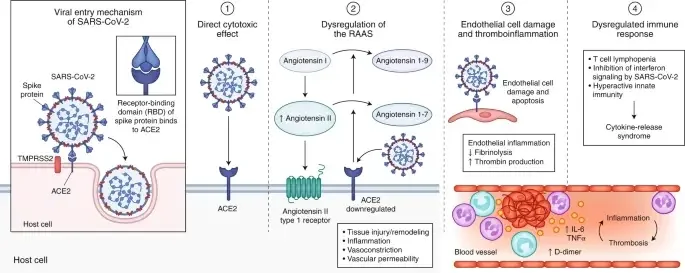
- The incubation period lasts between 1 and 14 days. Variation in the incubation period is connected with the age of patients, with those over 60 developing symptoms more rapidly than others.
- SARS-CoV-2 affects the alveolar epithelial cells. After the virus has entered the respiratory system, the cilia in the lower respiratory tract assist the virus’ attachment to its receptor.
- The mucus covering the respiratory epithelium is moved towards the throat by the cilia. This enables the virus’ adhesion.
- Multiple SARS-CoV-2 proteins interact with innate immune system components to avoid an interferon-mediated antiviral response.
- After SARS-CoV-2 infection, the expression of hACE2, the SARS-CoV-2 receptor, at the cell surface is downregulated.
- The mechanism of this downregulation appears to involve the internalisation of ACE2 during SARS-CoV entry and the stimulation of metalloprotease TNF-converting enzyme activity.
- This domain is lost as a result of the cleavage of the ACE2 extracellular domain from its transmembrane domain by the enzymes.
- This has led to the suggestion that the binding of SARS-CoV-2 S protein represents an additional virulence factor for SARS, in addition to its role in viral attachment and entry.
- Patients infected with COVID-19 have elevated leukocyte counts, aberrant respiratory signs, and elevated plasma pro-inflammatory cytokine concentrations.
- As a virus that targets the respiratory system, the primary pathogenesis of COVID-19 infection is severe pneumonia, RNAaemia, and the occurrence of acute cardiac injury.
- Patients with COVID-19 infection have significantly elevated blood levels of cytokines and chemokines, including IL1-β, IL1RA, IL7, IL8, IL9, IL10, basic FGF2, GCSF, GMCSF, IFNγ, IP10, MCP1, MIP1α, MIP1β, PDGFB, TNFα, and VEGFA.
- In addition, patients with severe symptoms have elevated levels of pro-inflammatory cytokines such as IL2, IL7, IL10, GCSF, IP10, MCP1, MIP1α, and TNFα, which are believed to exacerbate illness severity.
- The link between increasing clinical progression, dropping viral loads, and the initiation of an immunological response, along with the presence of significantly raised cytokine levels, suggests that severe lung damage is mostly immunopathological in nature.
Replication of Coronavirus (SARS-CoV-2)
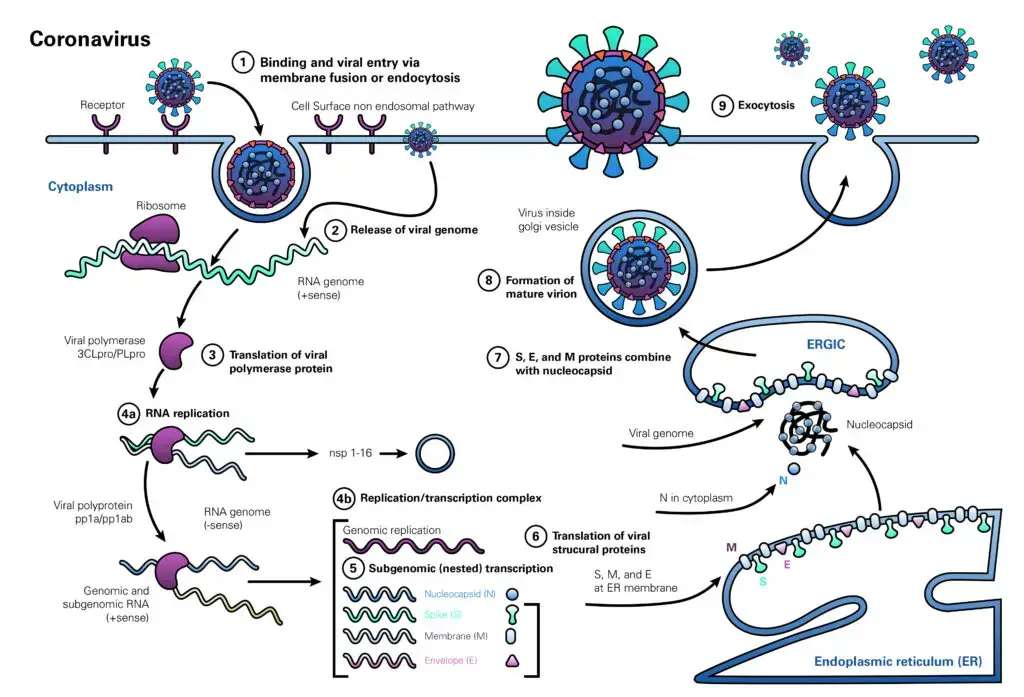
- The coronavirus life cycle begins when the virion attaches to the host cell receptor via the S1 subunit of its spike protein (step 1). The interaction between S-protein and receptor controls the virus’s host species range and tissue tropism.
- Numerous alpha-coronaviruses, for instance, utilise aminopeptidase N as their receptor, but SARS-CoV and HCoV-NL63 employ angiotensin-converting enzyme 2 (ACE2) as the host receptor. MHV uses CEACAM1 to enter human cells, whereas MERS-CoV binds dipeptidyl peptidase 4 (DPP4).
- The ensuing illness profile is highly dependent on the distribution of the receptor within human tissues.
- Following receptor attachment, the virus gains access to the cytosol through acid-dependent proteolytic cleavage of the S protein into S1 and S2 subunits by furin, cathepsin, TMPRSS2, or another protease, followed by S2-assisted fusion of the viral and cellular membranes. After viral genome release (step 2), genomic RNA is converted into replicase (step 3).
- The subsequent phase in viral RNA production (step 4a) is the construction of viral replication-transcription complexes (step 4b). The viral structural proteins (S, E, and M) are translated from RNA (step 5), inserted into the endoplasmic reticulum (step 6), and then transported to the endoplasmic reticulum-Golgi intermediate compartment (step 7). (ERGIC).
- Multiple copies of the nucleocapsid (N protein) wrap genomic RNA into helical structures (ribonucleoprotein complexes) in the cytoplasm and interact with hydrophobic M proteins (envelope protein) in the ERGIC to direct virion formation (step 7). Virions released from the ERGIC membranes (step 8) are subsequently carried out of the cell via the constitutive exocytic pathway (step 9).
References
- Caly L, Druce J, Catton M, Jans D and Wagstaff K. The FDA-approved Drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Research. 2020. 104787. ISSN 0166-3542, https://doi.org/10.1016/j.antiviral.2020.104787. (http://www.sciencedirect.com/science/article/pii/S0166354220302011)
- Kaye M. (2006). SARS-associated coronavirus replication in cell lines. Emerging infectious diseases, 12(1), 128–133. https://doi.org/10.3201/eid1201.050496
- Weiss, S. R., & Navas-Martin, S. (2005). Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiology and molecular biology reviews : MMBR, 69(4), 635–664. https://doi.org/10.1128/MMBR.69.4.635-664.2005
- Weiss SR and Leibowitz JL. Chapter 4 – Coronavirus Pathogenesis. Advances in Virus Research. Academic Press. Volume 81. 2011. Pages 85-164. ISSN 0065-3527. https://doi.org/10.1016/B978-0-12-385885-6.00009-2.
- Guo Y, Cae Q, Hong Z, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Millitary Medical Reasearch. (2020) 7:11. https://doi.org/10.1186/s40779-020-00240-0
- Fehr, A. R., & Perlman, S. (2015). Coronaviruses: an overview of their replication and pathogenesis. Methods in molecular biology (Clifton, N.J.), 1282, 1–23. https://doi.org/10.1007/978-1-4939-2438-7_1
- Ou X, Liu Y, Lei X, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nature Communications (2020) 11:1620 | https://doi.org/10.1038/s41467-020-15562-9
- https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions
- https://microbenotes.com/transmission-pathogenesis-replication-of-sars-cov-2/
- https://www.wikidoc.org/index.php/Coronavirus_pathophysiology
- https://www.lsbio.com/media/whitepapers/sars-cov-2-and-covid-19-pathogenesis-a-review#biology-and-life-cycle
- https://www.bmj.com/content/371/bmj.m3862#:~:text=Viral%20entry%20and%20interaction%20with,%2C%20headache%2C%20and%20respiratory%20symptoms.
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8311250/
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.