What are the Romanowsky Stains?
Romanowsky stains are the polychromatic stains that are used in hematological and cytological studies to differentiate blood cells and bone marrow cells under the microscope. It is the process where a mixture of acidic dye (Eosin Y) and basic dyes (oxidized methylene blue or Azure B) is applied, and this mixture produces the characteristic Romanowsky effect. This effect gives rise to different coloration such as purple, blue, and pink because the dyes react with different cellular components. The nucleus, cytoplasm, and granules therefore appear in different shades, and this helps in identifying the cells. It is this staining method that also detects parasites present in the blood like Plasmodium species causing malaria, and organisms such as Leishmania and Trypanosoma in blood films.
The Romanowsky stains are named after Dmitri Romanowsky, who first demonstrated the usefulness of combining these dyes to show cell structures clearly. It is the reaction of Azure B and Eosin that produces the purple coloration seen in chromatin and in some neutrophil granules. These stains have several types that all apply the same staining principle.
Some of the important types are Giemsa stain, Wright and Wright–Giemsa stain, May–Grünwald stain and Leishman stain. These stains are used in differential leukocyte count, assessment of red blood cell morphology, and identifying abnormal cells in cases such as leukemia and anemia. They are also used in cytopathology to examine body fluid samples like pleural fluids for abnormal or malignant cells.
Principle of Romanowsky Stains
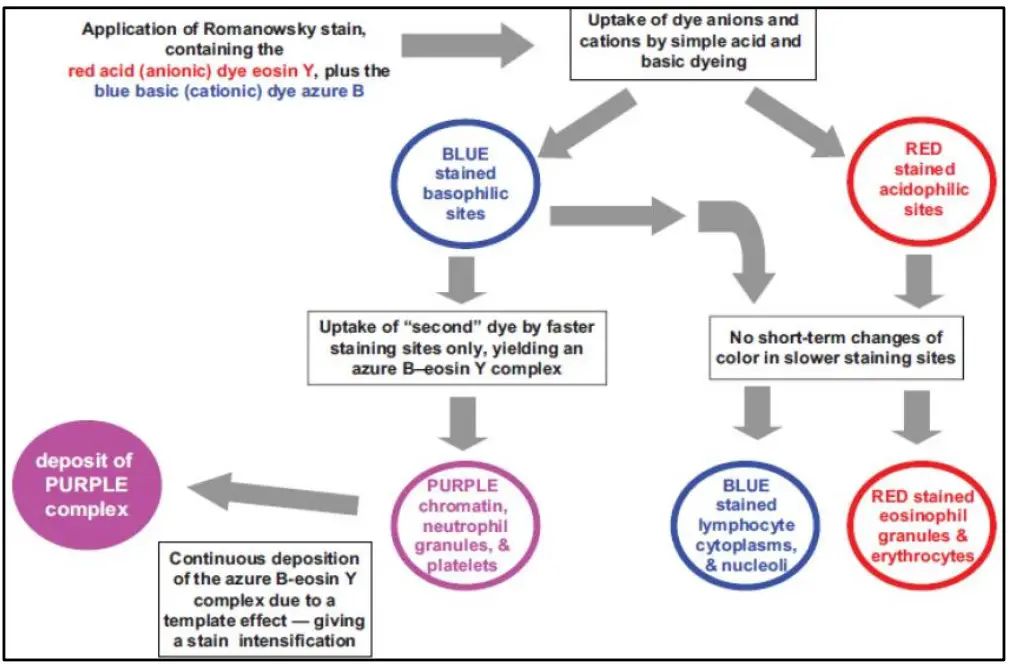
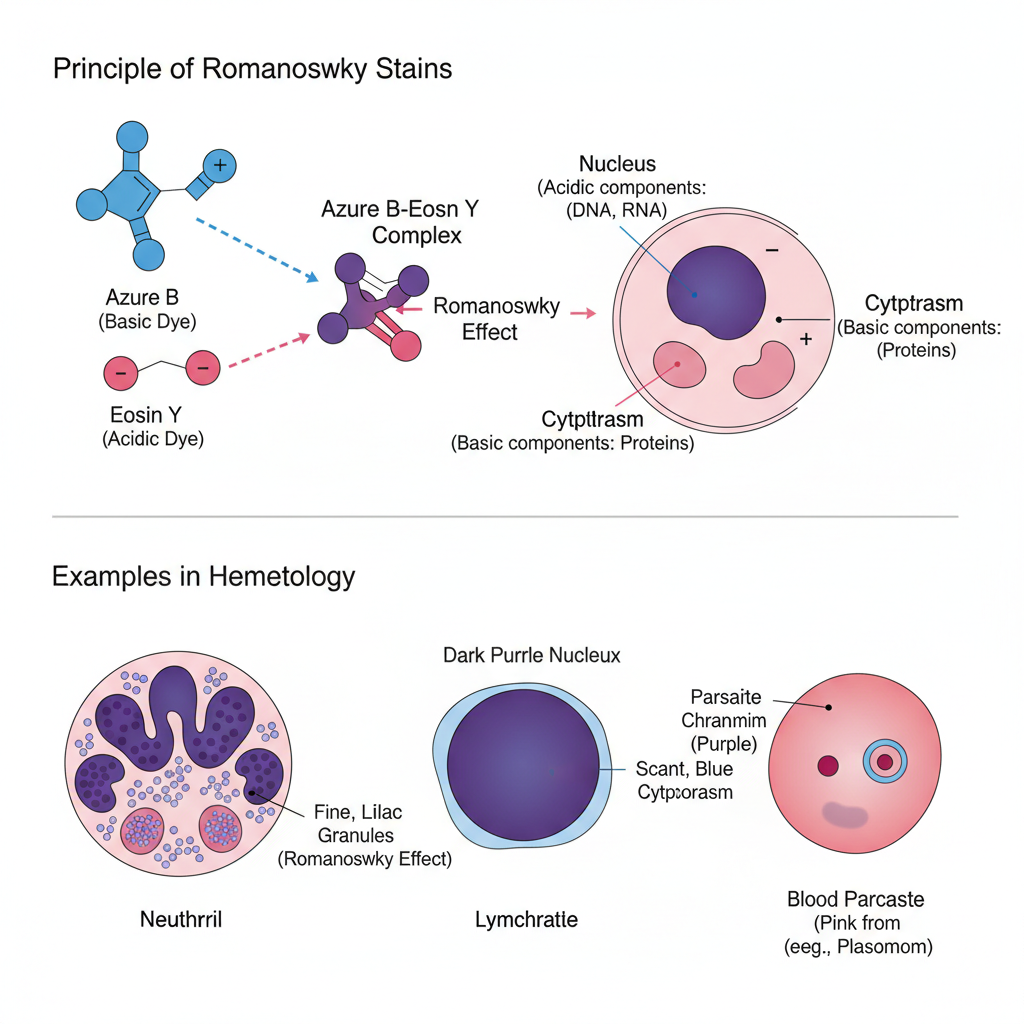
The principle of Romanowsky stains is based on the combined action of acidic and basic dyes that interact with different cellular components to produce a characteristic polychromatic staining effect. It is the oxidized methylene blue (Azure B) which acts as the basic dye, and Eosin Y which acts as the acidic dye, and both bind to structures according to their chemical nature. The basic dye binds with acidic nuclear materials such as DNA and RNA, producing blue to purple coloration, while the acidic dye binds with alkaline cytoplasmic components including hemoglobin, giving red or pink coloration. It is the chemical interaction of Azure B and Eosin that forms a complex which produces the typical purple color seen in chromatin and in neutrophil granules.
This phenomenon is referred to as the Romanowsky effect. This is the process that helps in distinguishing the nucleus, cytoplasm, and granules clearly, making the stain useful in blood cell differentiation and detection of blood parasites.
Types of Romanowsky Stains
Types of Romanowsky Stains are classified according to the stains that apply the Romanowsky principle and produce the characteristic polychromatic effect. These stains use mixtures of methylene blue derivatives and eosin dyes to differentiate blood cells and parasites. Some of the main types are–
- Wright’s stain – It is formed by heating methylene blue to make polychromed methylene blue and mixing it with Eosin Y. It stains blood films with balanced blue and pink coloration and is used for routine peripheral blood and bone marrow studies.
- Giemsa stain – It contains Azure II (Azure B + methylene blue) with Eosin Y in glycerol and methanol. It is used for detection of parasites like Plasmodium, Leishmania and Trypanosoma and also for bone marrow staining.
- Wright–Giemsa stain – It is the combined formulation of Wright and Giemsa stains. It gives intense basophilic staining and is widely applied for white blood cell morphology and for detecting parasites.
- May–Grünwald–Giemsa stain (MGG) – It uses May–Grünwald stain followed by Giemsa stain. It is used in cytology such as FNAC smears and lymph node impressions, giving detailed cytoplasmic granule staining.
- Leishman stain – It contains polychrome methylene blue and Eosin dissolved in methanol. It is used for identification of WBCs and parasites like malaria and trypanosomes.
- Field’s stain – It uses two solutions, Field A (methylene blue and azure) and Field B (eosin). It is used for rapid staining of thick blood films, mainly for malaria diagnosis in field settings.
- Diff–Quik stain – It is a rapid commercial modification of the Romanowsky method using a fixative and two stain solutions. It is used for quick cytology assessment and special tests like sperm vitality.
- Jenner stain – It contains eosin and methylene blue in alcohol. It does not produce the complete Romanowsky effect but was an early formulation leading to modern stains.
- Modified Ultrafast Giemsa (MUFG) – It uses undiluted Giemsa stock solution for rapid cytology evaluation especially during onsite assessments, staining within a few minutes.
Factors affecting staining
- pH of the staining buffer – It is the main factor controlling dye binding. When the pH is below 6.8 the eosin binds more and produces excessive red staining, and nuclei appear pale. When the pH is above 6.8 the basic dyes bind more and produce excessive blue staining and the red blood cells show a gray-blue colour. The optimum pH is around 6.8 for balanced staining.
- Fixation quality and moisture – Methanol is used to fix the smear, and proper fixation is needed to preserve cell morphology. Delay in fixation or insufficient fixation time leads to poor staining and bluish background. Moisture in the fixative or on the slide can produce cloudiness and a dirty background.
- Staining time and stain concentration – If the smear is exposed for a short time the staining becomes weak leaving pale nuclei and pale cytoplasm. Longer exposure produces overstaining. The staining takes place in stain mixed with buffer, and the stain–buffer ratio affects the final intensity. Inadequate rinsing leaves precipitates on the slide.
- Dye composition and polychroming – The Romanowsky effect is produced when Eosin Y reacts with Azure B. Azure B forms from oxidative changes of methylene blue. If oxidation is insufficient the characteristic purple colour is not formed. The dye mixtures are unstable and can deteriorate in air or light, making the stain less intense.
- Smear preparation and thickness – Thick smears retain too much dye and stain unevenly, often giving a heavy blue background. The smear must be air-dried quickly to maintain cell size and granules. Slow drying or moisture distortion affects the appearance of the cells and reduces staining clarity.
Troubleshooting of Romanowsky Stains
| Too PINK | • Improper azure B/eosin Y ratio • Impure dye • Low pH |
| Too BLUE | • Improper azure B/eosin Y ratio • Stock stain exposed to light • Excess staining time • Thick film • Inadequate time in buffer solution |
| Too PALE | • Old solution • Weak/Impure dyes • High temperature |
| Nuclei too dark | • Stain too concentrated • Incorrect staining time |
Applications of Romanowsky Stains
These are mainly applied in different diagnostic fields. The major uses are–
Hematology
- It is used for differential blood cell counts where WBC types are identified in peripheral blood smears.
- It helps in observing RBC morphology and detection of polychromasia which indicate increased RBC production.
- Bone marrow smears is examined for cellularity and maturation and identification of abnormal cells in leukemia.
- Diagnosis of hematological disorders is done by detecting abnormal cells in anemia and infections.
- It is also used for allergen testing because eosinophils show intense red staining in allergic responses.
Parasitology and Microbiology
- Malaria parasites is identified in thin and thick blood films as Giemsa stain is considered the gold standard.
- Other bloodborne parasites like Trypanosoma, Babesia and Leishmania is detected in blood or tissue smears.
- Bacteria like Helicobacter pylori, Borrelia, Yersinia pestis and Chlamydia trachomatis inclusion bodies is stained.
- It helps in identifying fungal organisms such as Histoplasma and Pneumocystis jiroveci cysts.
Cytopathology
- Fine-needle aspiration air-dried smears is examined from lymph nodes, thyroid, breast and salivary glands to detect benign or malignant lesions.
- Body fluids like pleural fluid, ascitic fluid, CSF and synovial fluid is examined for abnormal or malignant cells.
- Rapid on-site evaluation (ROSE) is possible because Diff-Quik or MUFG variants stain in under a minute for assessing adequacy during procedures.
Cytogenetics
- Chromosomal banding (G-banding) is done to detect chromosomal defects and structural abnormalities.
Advantages of Romanowsky Stains
- It is known for producing clear colour differentiation due to the Romanowsky effect which gives distinct purple, blue and pink shades helping in identification of nucleus, cytoplasm and granules.
- It is used as the major stain in hematology because it differentiates erythrocytes, platelets and leukocytes and it also helps in observing immature cells like reticulocytes.
- It is the main stain for detection of blood-borne parasites. The parasite nucleus stains deep purple or blue which make organisms like Plasmodium, Trypanosoma and Leishmania easy to identify.
- It is used in many diagnostic fields such as hematology, cytology, histology, bacteriology and cytogenetics and the stain is suitable for blood, bone marrow and other body fluids.
- It gives better cytoplasmic and stromal details and highlights metachromatic substances like mucins, colloid and lymphoglandular bodies.
- It is applied in cytogenetics for preparing karyograms because Giemsa stain reveals AT-rich and GC-rich chromosome regions (G-banding).
- It is simple to prepare and the reagents are easily available which make the staining procedure convenient.
- It allows rapid staining when using variants like Diff-Quik or MUFG as the stain completes in less than one minute and is useful for onsite evaluation.
- It is used on air-dried smears so the cell shrinkage seen in wet fixation is not present which gives a better estimation of the cell size and nuclear size.
Disadvantages of Romanowsky Stains
- It is known that Romanowsky stains show instability in their stock solution and there is batch-to-batch variation which results in inconsistent staining.
- It is highly sensitive to pH changes. When the buffer becomes alkaline (above pH 6.8) the smear becomes excessively blue with poor nuclear details and when the buffer becomes acidic the smear becomes overly red with pale nuclei.
- It is a time-taking procedure because classical methods like Giemsa or MGG need around 20–30 minutes and the preparation of buffer and working solution makes the process tedious.
- The cell morphology may be distorted in some preparations and in cytology the background often appears dirty. Cytoplasmic details may not be as clear because there is no clearing stage.
- Quick staining variants do not work well for bone marrow smear as they cannot penetrate the tissue in the short staining duration. MUFG stain also shows non-uniform staining in thick or hemorrhagic smears and heavy RBC staining can obscure the cell features.
- Precipitate formation is common when unfiltered or poorly rinsed stain is used which cause artifacts on the slide and these may mimic microorganisms.
FAQ
What are Romanowsky stains?
Romanowsky stains are differential blood stains that are made by combining acidic and basic dyes and it is used to colour blood cells and parasites on air-dried smears.
What are Romanowsky stains used for?
These stains are used mainly in hematology for observing erythrocytes, leukocytes, platelets and also for detecting blood-borne parasites on stained smears.
What is the principle of Romanowsky staining?
It is the process in which acidic dye and basic dye interact with cellular components producing a metachromatic effect and in this way the nuclei, cytoplasm and granules take different colours.
What are the different types of Romanowsky stains?
Some of the main stains are– Giemsa stain, Wright stain, Leishman stain, May-Grünwald stain and several rapid variants prepared from these dyes.
How do Romanowsky stains work?
The dyes bind to the chemical groups of the cell structures and the combination of azure dyes with eosin produces characteristic purple, blue and pink colours.
What is the Romanowsky effect?
It is the metachromatic property in which azure and eosin dyes form a complex that gives a purple shade to chromatin and some granules which help in differentiation of blood cells.
What dyes are found in Romanowsky stains?
The major dyes are methylene blue oxidation products (azure A and azure B) and eosin Y which together give the Romanowsky effect.
Which Romanowsky stain is used for malaria diagnosis?
Giemsa stain is used for diagnosis of malaria because the parasite nucleus stains purple or blue making it easy to identify.
Who invented Romanowsky stains?
It is introduced by Dmitri Romanowsky in the late 19th century when he observed the metachromatic staining produced by azure and eosin mixtures.
Why is buffer important in Romanowsky staining?
The buffer maintains the required pH for dye binding and if the pH changes slightly the smear becomes too blue or too red which affect the visibility of nuclei and cytoplasm.
What are common troubleshooting issues with Romanowsky stains?
Some of the common issues are formation of precipitates on slides, non-uniform staining, dirty background, excessive blue or excessive red staining caused by wrong pH and problems in stain penetration in thick smears.
What are the advantages of using Romanowsky stains?
Some of the advantages are– it gives clear colour differentiation, it is important for hematological diagnosis, it is used for detecting parasites, it shows good cytoplasmic details and it is applicable in several diagnostic fields.
What are the disadvantages of Romanowsky stains?
These stains show instability, they are very sensitive to pH, they require time and careful preparation, they may distort morphology, some variants do not stain bone marrow well and precipitates may form on the slide.
Is Diff-Quik considered a Romanowsky stain?
Diff-Quik is a rapid variant derived from Romanowsky principles and it is based on similar dye combinations.
How do you prepare a Romanowsky stain?
It is prepared by dissolving methylene blue dye and eosin dye in suitable solvents, allowing methylene blue to oxidise to azure forms, then mixing with buffer of correct pH and filtering the working solution before staining.
- Astral Diagnostics. (n.d.). Lab technician’s guide to troubleshooting: 20 common issues with biological stains [E-book].
- Atom Scientific. (n.d.). Stains in focus: The development of the Romanowsky technique. Retrieved from https://atomscientific.com/news/stains-in-focus-the-development-of-the-romanowsky-technique
- Cleveland Clinic. (2023, July 13). Polychromasia. https://my.clevelandclinic.org/health/diseases/25128-polychromasia
- Deepthi, B., Prayaga, A. K., & Rukmangadha, N. (2022). Comparison of modified ultrafast Giemsa stain with the standard May Grunwald Giemsa stain in FNAC of various organs. Journal of Cytology, 39(4), 174–179. https://doi.org/10.4103/joc.joc_43_22
- Emanuel, P. (2013). Leishmaniasis pathology. DermNet. https://dermnetnz.org/topics/leishmaniasis-pathology
- Ethos Biosciences. (n.d.). Choosing between Wright’s stains or Wright-Giemsa stains. Retrieved from https://www.ethosbiosciences.com/choosing-wrights-or-wright-giemsa-stains
- Fasakin, K. A., Okogun, G. R., Omisakin, C., & Ayodele, E. (2014). Modified Leishman stain: The mystery unfolds. British Journal of Medicine & Medical Research, 4(27), 4591–4606.
- Horobin, R. W., & Walter, K. J. (1987). Understanding Romanowsky staining. I: The Romanowsky-Giemsa effect in blood smears. Histochemistry, 86(3), 331–336. https://doi.org/10.1007/BF00490267
- Jackson, S., Grabis, D., & Manav, C. (2018). Giemsa: The universal diagnostic stain [Poster]. MilliporeSigma. https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/193/146/ps2671en-mk.pdf
- Macsen Labs. (n.d.). Giemsa stain | Composition, principle, procedure & uses. https://www.macsenlab.com/blog/giemsa-stain-overview/
- Mokobi, F. (2022, September 5). Romanowsky stains- Principle, types, applications. Microbe Notes. https://microbenotes.com/romanowsky-stains/
- Nurhaliza, W., Rahmawati, Y., & Nailufar, Y. (2025). Literature review: Comparison of the quality of Papanicolaou, Giemsa, and May-Grunwald Giemsa staining in pleural effusion specimens. Jurnal Kesehatan STIKes Muhammadiyah Ciamis, 12(2), 180–188.
- Racelis, A. (2025, June 24). Polychromasia: What it is, what causes it, and how it’s treated. WebMD. https://www.webmd.com/a-to-z-guides/what-is-polychromasia
Romanowsky stain. (n.d.). In Wikipedia. Retrieved from https://en.wikipedia.org/wiki/Romanowsky_stain - The physico-chemical basis, variants, and diagnostic utility of Romanowsky stains. (2025)..
- Woronzoff-Dashkoff, K. K. (2002). The Wright-Giemsa stain – Secrets revealed. Clinics in Laboratory Medicine, 22(1), 15–23. https://doi.org/10.1016/S0272-2712(03)00065-9
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.