What is Robertson’s Cooked Meat Medium (RCM Medium)?
- Robertson’s Cooked Meat (RCM) Medium, also known as Cooked Meat Broth (CMB), serves as a pivotal substrate for the cultivation of a diverse range of microorganisms, with a primary focus on anaerobic and aerobic bacteria. This medium finds particular utility in isolating pathogenic Clostridium species derived from various sources, including clinical, food, and water samples. Furthermore, RCM Medium doubles as a proficient maintenance medium for stock cultures of both anaerobic and aerobic microorganisms.
- At its core, RCM Medium comprises fat-free minced cooked meat sourced from ox heart, combined with a nutrient-rich broth. This unique composition renders it an ideal medium for fostering the growth of a spectrum of microorganisms, including spore-forming and non-spore-forming obligate anaerobes. The inclusion of cooked meat pieces plays a crucial role in reducing oxygen levels within the culture, a necessary condition for the propagation of anaerobic microorganisms.
- The medium’s versatility extends to its ability to cultivate aerobic, microaerophilic, and anaerobic microorganisms, making it a valuable tool in microbiological laboratories. To achieve anaerobic conditions, various agents, such as glucose, thioglycollate, cysteine, and ascorbic acid, are employed to diminish the oxygen content in the culture media. Thioglycollate broth, containing nutrient broth and 1% thioglycollate, represents another medium used for cultivating anaerobes.
- Researchers often employ RCM Medium as an enrichment medium to revive organisms present in low numbers on the original swab, which might be lost during transportation before direct culture in the laboratory. Additionally, it aids in the detection of toxin production and facilitates the demonstration of saccharolytic and proteolytic properties in Clostridia.
- For those engaging in Gas Liquid Chromatography examinations of anaerobic isolates, a variant of RCM containing glucose and vitamin K is recommended. This variant proves valuable in preserving anaerobes for subsequent analysis.
- Despite the advantages of RCM Medium, challenges arise due to the simultaneous proliferation of aerobes alongside anaerobes in the culture. This potential oversight in isolating anaerobes is mitigated by the addition of Neomycin to the medium. The modified N.RCM not only ensures successful isolation but also allows for the evaluation of its efficacy by comparing results with routine RCM. This augmentation enhances the medium’s utility in clinical settings, where early qualitative results can provide crucial insights for clinicians, albeit with a cautionary note about potential limitations in quantitation due to the concurrent growth of aerobes.
Principle of Robertson’s Cooked Meat Medium (RCM Medium)
- The principle underlying Robertson’s Cooked Meat (RCM) Medium is grounded in its meticulously crafted composition, each component serving a specific function to facilitate the cultivation of anaerobic and aerobic bacteria, particularly focusing on pathogenic Clostridium species. The intricate balance of components contributes to the medium’s efficacy, and a detailed examination reveals the sequential functions of its constituents.
- Firstly, HMH peptone B, an integral component derived from cooked meat, plays a pivotal role by providing essential amino acids and nutrients. Moreover, it contains glutathione, a reducing substance crucial for the growth of obligate anaerobes. The sulfhydryl groups within denatured protein, particularly abundant in cooked meat, impart a significant reducing effect, creating an environment conducive to the cultivation of anaerobes.
- The inclusion of glucose (dextrose) in the medium serves a dual purpose. It not only allows for the rapid and robust growth of anaerobic bacteria within a short timeframe but also facilitates the swift identification of key anaerobic microorganisms. Sodium chloride (NaCl) is strategically added to the medium to permit adjustments in osmotic pressure, ensuring an optimal environment for bacterial growth.
- The indicators of growth in RCM Medium are manifested through observable changes. Turbidity or bubble formation indicates the presence and proliferation of certain organisms. However, proteolysis, the decomposition of meat, results in the formation of foul-smelling sulfur compounds and blackening. Saccharolytic strains may produce hydrogen sulfide, causing a lesser degree of blackening. Additionally, some anaerobes exhibit rapid acid and gas production without digesting the meat, resulting in slightly sour-smelling cultures with reddened protein.
- For optimal results, it is imperative to use the medium on the day of preparation. Alternatively, if not promptly utilized, the medium should undergo a brief boiling or steaming process, followed by cooling without agitation, before inoculation. Inoculation is strategically performed near the bottom of the tube in the meat particles for anaerobic cultures, as aerobes tend to grow at the top, while more anaerobic species thrive deeper in the medium.
- The preparation of the medium involves a careful process to maintain an oxygen-free environment. Prior to inoculation, the RCM/CMB medium undergoes boiling, and post-inoculation, it is shielded with a layer of sterile liquid paraffin oil to prevent the ingress of oxygen, ensuring the desired anaerobic (reduced) conditions.
- Furthermore, the reducing agents present in meat, such as glutathione and cysteine, actively utilize oxygen, contributing to a reduced oxidation-reduction potential. The unsaturated fatty acids in meat participate in auto-oxidation, a reaction catalyzed by haematin. This intricate interplay of components underscores the medium’s ability to create and sustain an environment conducive to the growth of specific microorganisms.
- Originally developed by Robertson for the cultivation of anaerobes isolated from wounds, RCM Medium’s applications have expanded. It is recommended by BIS for the detection and enumeration of bacteria responsible for food poisoning, especially Clostridium welchii. With slight modifications, it serves as a salt medium for the isolation of Staphylococci. The FDA has also endorsed a modified version for the enumeration and identification of Clostridium perfringens from foods. In essence, the principle of Robertson’s Cooked Meat Medium lies in its finely tuned composition, fostering a selective environment for the cultivation and study of specific bacteria.
Composition of Robertson’s Cooked Meat Medium (RCM Medium)
- Cooked Meat Medium (CMM): The primary component, contributing 250.0 gm per liter, is the cooked meat medium. In RCM Medium, meat particles play a crucial role as a reducing and detoxifying substance. Their function is to disable harmful by-products that may be generated during the replication of microorganisms. The cooking process is essential, as it denatures the protein in the meat, making reducing substances more available.
- Peptic Digest of Animal Tissue: This ingredient, present at 17.5 gm per liter, serves as a nutritional supplement. It provides the necessary nutritional requirements for the majority of bacteria, contributing to the growth and vitality of the microbial cultures within the medium.
- Dextrose: At 5.0 gm per liter, dextrose serves as another nutritional supplement, providing a source of glucose. Glucose facilitates the rapid and robust growth of anaerobic bacteria within a short timeframe, aiding in the swift identification of key anaerobic microorganisms.
- Sodium Chloride: This component, added at 5.0 gm per liter, allows for adjustments in osmotic pressure. It ensures that the medium maintains an optimal environment for bacterial growth by regulating the concentration of solutes.
- Yeast Extract: Contributing 5.0 gm per liter, yeast extract is another nutritional supplement providing essential nutrients for bacterial growth. It complements the overall nutritional profile of the medium.
- Iron Filings: Included at 10.0 gm per liter, iron filings serve as a reducing substance. The combination of iron filings and muscle tissue permits the growth of strict anaerobes, emphasizing the medium’s selectivity for anaerobic microorganisms.
- Hemin and Vitamin K: Both hemin and vitamin K are added at 10.0 ml each per liter to enhance the growth of anaerobic microorganisms. These supplements play a crucial role in creating a supportive environment for the specific bacteria targeted by RCM Medium.
The final pH of the medium is adjusted to 6.8 +/- 0.3 at 25ºC, ensuring a stable and optimal pH for microbial growth. It’s important to note that the composition may be adjusted or supplemented as required to meet specific performance criteria, highlighting the adaptability of RCM Medium for various microbiological applications.
| Cooked Meat Medium | 250.0 gm |
| Peptic Digest of Animal Tissue | 17.5 gm |
| Dextrose | 5.0 gm |
| Sodium Chloride | 5.0 gm |
| Yeast Extract | 5.0 gm |
| Iron Filings | 10.0 gm |
| Hemin | 10.0 ml |
| Vitamin K | 10.0 ml |
Final pH 6.8 +/- 0.3 at 25ºC.
* Modified and/or added according to the need to meet requirements for performance
- Cooked Meat Medium: In the cooked medium, meat particles function as a detoxifying and reducing substance, thus preventing harmful by-products created in the organism that reproduces. Since reducing substances are accessible in protein denatured which is why it is cooked prior to being used for the medium.
- Iron filings: Reducing substance. The iron filings and the muscle tissue facilitate the expansion of strict anaerobes.
- Nutritional supplements: Nutritional needs of the majority of bacteria can be met through peptic digests of tissues from animals, yeast extract and dextrose. Hemin as well as vitamin K can be included to increase the development of anaerobic microorganisms. The amino acids as well as other nutrients are also provided by the muscles protein found in the granules of heart tissue.
Preparation of the RCM Medium
The preparation of Robertson’s Cooked Meat (RCM) Medium is a systematic process, best achieved using ready-to-use dehydrated granules readily available from culture media suppliers.
- Dispensing Granules:
- Utilizing a small tube or scoop pre-marked to hold 1g of granules, the medium is dispensed in 1g amounts into screw-cap bottles or tubes. This precision ensures the accurate formulation of the medium.
- Adding Distilled Water:
- Subsequently, 10 ml of distilled water is added to the granules. This step initiates the rehydration process, allowing the granules to absorb water and begin the transformation into the liquid medium.
- Mixing and Soaking:
- The mixture is thoroughly mixed, and the components are allowed to soak for 5 minutes. This soaking period facilitates the complete absorption of water by the granules, ensuring the proper reconstitution of the medium.
- Autoclaving:
- Following the mixing and soaking period, the medium is sterilized by autoclaving. During this process, the caps of the bottles or tubes are loosened to allow for the escape of air and prevent excessive pressure buildup. Autoclaving is conducted at 121°C for 15 minutes, effectively eliminating any contaminants and preparing the medium for use.
- Cooling and Tightening Caps:
- Once autoclaving is complete, the medium is allowed to cool to room temperature. Subsequently, the bottle caps are tightened, sealing the medium to maintain its sterility. This step is crucial to prevent any potential contamination post-sterilization.
- Labeling and Batch Number:
- The prepared medium is labeled with the date of preparation and assigned a batch number for traceability and quality control purposes. This information helps in monitoring the medium’s shelf life and ensuring its proper usage.
- Storage:
- The medium is stored in a cool, dark place, with an emphasis on tightly screwed bottle caps to maintain its sterility. Proper storage conditions are crucial for preserving the medium’s integrity and effectiveness.
- Shelf-Life:
- The shelf-life of the prepared RCM Medium is determined by its volume and appearance. As long as there is no change in these parameters to suggest contamination, the medium retains its efficacy for up to 2 years.
- pH Adjustment:
- The pH of the prepared medium is a critical factor, and it should fall within the range of pH 7.0-7.4 at room temperature. This ensures the medium’s compatibility with the targeted microorganisms and optimal conditions for bacterial growth.
Quality Control of Robertson’s Cooked Meat Medium
The quality control of Robertson’s Cooked Meat (RCM) Medium involves a comprehensive assessment of various parameters, ensuring its suitability for the cultivation of specific microorganisms. The evaluation encompasses the appearance, color and clarity of the prepared medium, reaction, pH, and cultural response.
- Appearance:
- The quality control process begins with the assessment of the appearance of RCM Medium. The medium is in the form of brown-colored granules. This characteristic visual aspect provides an initial indicator of the medium’s integrity.
- Color and Clarity of Prepared Medium:
- After preparation, the color and clarity of the medium are evaluated. The prepared medium exhibits a medium amber-colored appearance with a clear to slightly opalescent supernatant over insoluble granules. This observation ensures that the reconstitution process and sterilization have occurred effectively, maintaining the desired characteristics of the medium.
- Reaction:
- The reaction of the medium is determined by assessing a 11.54% w/v aqueous suspension at 25°C. The pH is specified to be 7.8±0.2. This step is crucial as it verifies the maintenance of the intended pH range, which is fundamental for the optimal growth conditions of targeted microorganisms.
- pH:
- The pH of the RCM Medium is expected to fall within the range of 7.60-8.00. This parameter is vital for ensuring the compatibility of the medium with the microorganisms intended for cultivation. The specified pH range reflects the medium’s ability to support the growth of anaerobic and aerobic bacteria.
- Cultural Response:
- The cultural response involves the observation of specific microorganisms after incubation at 35°C for 40-48 hours. The organisms tested include Clostridium botulinum ATCC 25763, Clostridium perfringens ATCC 12924, Clostridium sporogenes ATCC 11437, Enterococcus faecalis ATCC 29212, and Streptococcus pneumoniae ATCC 6303.
- Growth:
- The growth of the tested organisms is evaluated and categorized based on their luxuriance. For the organisms mentioned, the expected cultural response is 50-100 luxuriant growth. This standardized assessment ensures that the medium is effective in supporting the proliferation of specific microorganisms.
Test Requirements Robertson’s Cooked Meat (RCM) Medium
- Test Sample:
- The process begins with the collection of the test sample, which could be water, food, or clinical specimens. This sample serves as the source of microorganisms that will be cultivated and studied using the RCM Medium.
- Robertson’s Cooked Meat (RCM) Medium:
- The central component of the test is the RCM Medium itself. This medium, comprised of specific biological components such as cooked meat particles, peptic digest of animal tissue, dextrose, sodium chloride, yeast extract, iron filings, hemin, and vitamin K, creates an environment suitable for the growth of anaerobic and aerobic bacteria.
- Inoculating Loop (Optional):
- Depending on the nature of the specimens, an inoculating loop may be used. This tool aids in the transfer of a small, precise amount of the test sample onto the RCM Medium, ensuring controlled inoculation.
- Bunsen Burner:
- The Bunsen burner is employed to create a sterile environment during the inoculation process. By producing an open flame, it serves to sterilize the inoculating loop, minimizing the risk of contamination.
- Incubator:
- After inoculation, the medium-containing tubes or plates are placed in an incubator. The incubator provides controlled conditions, such as temperature and humidity, promoting the growth of microorganisms over a specified incubation period.
- Water Bath/Steamer (Optional):
- A water bath or steamer may be used as an optional tool, depending on the specific requirements of the test. These devices can aid in maintaining the temperature needed for certain microbiological processes.
- Control Strains (Positive and Negative Control):
- Positive and negative control strains are essential for validating the reliability and accuracy of the test. Positive controls consist of known microorganisms that should grow under the test conditions, confirming the medium’s efficacy. Negative controls, on the other hand, should not exhibit growth, providing a baseline for comparison.
Test Procedure of Robertson’s Cooked Meat (RCM) Medium
Anaerobic Culture
- Medium Preparation:
- Freshly prepared RCM Medium is preferred for anaerobic cultures. Inoculation is conducted as soon as the medium cools to approximately 35°C.
- Oxygen Removal for Stored Tubes:
- If RCM tubes are not used on the day of preparation, they should be subjected to a boiling water bath or steamer for about 15 minutes. This process eliminates dissolved oxygen. Afterward, the tubes are allowed to cool without agitation.
- Inoculation:
- Inoculation is performed near the bottom of the tube, specifically in the meat particles. This targeted inoculation ensures the growth of anaerobic microorganisms in the desired region of the medium.
- Incubation:
- Incubation is carried out for up to 7 days at 35°C with loosened caps. The loosening of caps allows for controlled aeration during the incubation period.
- Monitoring and Subculturing:
- The medium is examined daily for changes. Films and subcultures are made at intervals to monitor the growth and characteristics of microorganisms.
Aerobic Culture
- Inoculation Methods:
- Depending on the specimen, the cooked meat medium is inoculated using a swab, Pasteur pipette, or wire loop. If a swab is used, it should be inserted to the bottom of the container to ensure an effective transfer of microorganisms.
- Incubation for Aerobic Organisms:
- For the culture of aerobic organisms, the incubation is performed at 35°C with loosened caps. The incubation period extends for up to 7 days.
Strict Anaerobic Culture
- Fresh or Oxygen-Free Medium:
- For the culture of strict anaerobes, the medium is best used freshly prepared. Alternatively, if stored, it can be subjected to a boiling water bath or steamer to eliminate dissolved oxygen or placed in a water bath at 80°C for 30 minutes to ensure an oxygen-free state.
- Cooling and Inoculation:
- After oxygen removal, the medium is allowed to cool to room temperature before inoculation. The surface of the medium may be covered with a layer of sterile liquid paraffin to maintain anaerobic conditions.
Result and Interpretation of Robertson’s Cooked Meat (RCM) Medium
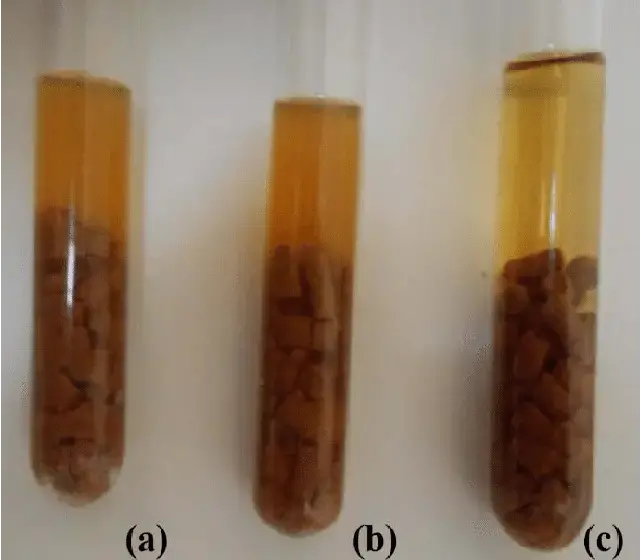
- Turbidity:
- The presence of turbidity in the medium indicates the growth of microorganisms. Turbidity is a visual indicator of microbial proliferation within the RCM Medium.
- No Turbidity:
- The absence of turbidity signifies no growth. If a no-growth scenario is reported, further investigation is recommended. The medium should be blindly subcultured into blood agar and incubated aerobically and anaerobically to confirm the absence of growth.
- Clostridium Species Classification:
- Clostridium species can be classified into two main groups based on their action on the medium:
- (i) Saccharolytic Group: Rapid production of acid and gas without digestion of the meat. May have a slightly sour smell with reddened protein.
- (ii) Proteolytic Group: Proteolysis causes meat decomposition, leading to foul-smelling sulfur compounds and blackening. Some saccharolytic strains may also produce hydrogen sulfide, causing blackening to a lesser degree.
- Clostridium species can be classified into two main groups based on their action on the medium:
- Aerobic and Anaerobic Growth:
- The medium is incubated with the cap loose, allowing aerobes to grow at the top and more anaerobic species to grow deeper in the medium.
- Cultural Characteristics:
- After incubation at 35-37°C for 40-48 hours, cultural characteristics are observed, and inoculum size is considered (50-100 CFU). Positive controls, including Clostridium botulinum, Clostridium tetani, Clostridium sporogenes, Enterococcus faecalis, and Streptococcus pneumoniae, are expected to show luxuriant growth.
- Clostridium perfringens:
- Saccharolytic anaerobes that turn the color of meat pieces into red.
- Clostridium tetani:
- Proteolytic anaerobes causing blackening of the meat.
- Positive Controls – Expected Results:
- Clostridium histolyticum and Clostridium perfringens show luxuriant growth with specific metabolic activities (proteolysis and saccharolysis).
- Negative Control:
- Uninoculated medium should exhibit no change.
- Saccharolytic and Proteolytic Reactions:
- Saccharolytic reactions show reddening of the meat with a rancid smell due to carbohydrate decomposition. Proteolytic reactions result in blackening of the meat with unpleasant smells due to protein decomposition.
| Organism | Growth |
| Clostridium botulinum | luxuriant |
| Clostridium perfringens | luxuriant |
| Clostridium sporogenes | luxuriant |
| Enterococcus faecalis ATCC | luxuriant |
| Streptococcus pneumoniae | luxuriant |
Limitations of Robertson’s cooked meat medium
- Freshness Requirement:
- For optimal results, Robertson’s Cooked Meat (RCM) Medium is best utilized on the day of preparation. The efficacy of the medium may diminish if not used promptly. To counter this limitation, it is recommended to either prepare a fresh batch for each use or subject stored tubes to a brief heating process before cooling and inoculation.
- Preparation Consideration:
- The medium should undergo a cooking or steaming process for a few minutes if not used on the day of preparation. This additional step is essential to eliminate any potential contaminants and dissolved oxygen, ensuring the medium’s suitability for cultivating anaerobic microorganisms.
- Cooling Process:
- After cooking or steaming, the medium should be allowed to cool without agitation before the inoculation process. This cooling step is crucial to prevent the introduction of contaminants and to create a stable environment for microbial growth.
- Inoculation Specifics:
- Inoculation should be performed near the top of the tube, specifically in the meat particles, especially for anaerobic strains. While this targeted inoculation enhances the growth of anaerobic microorganisms, it may limit the representation of aerobic species that tend to grow towards the top of the medium.
Uses of Robertson’s cooked meat medium
- Cultivation of Diverse Microorganisms:
- Robertson’s Cooked Meat (RCM) Medium serves as a versatile medium for the cultivation of aerobic, microaerophilic, and anaerobic microorganisms, with a particular emphasis on supporting the growth of Clostridium species. Its formulation is tailored to accommodate both spore-forming and non-spore-forming obligate anaerobes, making it a valuable tool in microbiological research.
- Support for Anaerobic Organisms:
- The medium’s design is especially beneficial for the cultivation of anaerobic microorganisms, providing an environment conducive to their growth. This is crucial for the isolation and study of organisms that thrive in low-oxygen conditions.
- Enrichment Broth for Small Inocula:
- Robertson’s Cooked Meat Medium is recognized for its utility as an enrichment broth, particularly valuable when dealing with very small inocula. This characteristic makes it effective in resuscitating organisms present in minimal quantities, enhancing the chances of successful cultivation.
- Preservation of Viability:
- Researchers have noted the medium’s effectiveness in preserving the viability of organisms over an extended period. This feature is particularly advantageous in maintaining anaerobic stock cultures, ensuring the long-term availability of specific microbial strains for experimental purposes.
- Recommendation by FDA for Food Analysis:
- The Food and Drug Administration (FDA) recommends the use of RCM for the enumeration and identification of Clostridium perfringens from food samples. This endorsement underscores its suitability for food microbiology applications, where the detection and analysis of specific bacterial species, such as Clostridium perfringens, are crucial for food safety assessments.
Important Notes on Robertson’s Cooked Meat (RCM) Medium
- Stock Culture Maintenance:
- For the maintenance of stock cultures, it is advised to keep the growth tube at room temperature after the initial incubation at 35°C. Subculturing should be performed every 4-6 months to ensure the vitality of the cultures.
- Storage Conditions and Shelf Life:
- The dehydrated medium should be stored at temperatures between 10-30°C and used before the specified expiry date on the label. Prepared medium, kept in the dark with tightly sealed caps, can be stored at room temperature for up to 6 months.
- Versatility for Aerobic and Anaerobic Organisms:
- Robertson’s Cooked Meat (RCM) Medium serves as an excellent medium for the primary growth and maintenance of both aerobic and anaerobic organisms. This versatility makes it valuable for a wide range of microbiological applications.
- Appearance of Prepared Medium:
- The color and clarity of the prepared medium are characterized by an amber hue, appearing clear to slightly opalescent in the supernatant over insoluble granules.
- Cultivation of Microorganisms:
- RCM is specifically designed for the cultivation of aerobic, microaerophilic, and anaerobic microorganisms, with a focus on Clostridia. It supports the growth of both spore-forming and non-spore-forming obligate anaerobes.
- FDA Recommendation for Clostridium perfringens:
- The U.S. Food and Drug Administration (FDA) recommends the use of RCM for the enumeration and identification of Clostridium perfringens from food samples, highlighting its suitability for food microbiology applications.
- Recovery Properties and Mixed Cultures:
- The excellent recovery properties of RCM may lead to mixed cultures resulting from sample inoculation. This is an important consideration in the interpretation of results, especially in scenarios where diverse microorganisms are present.
- Factors Influencing Medium Characteristics:
- The occurrence of blackening in the medium is contingent on the pH, and it will not occur in acidic conditions. Carbohydrate fermentation has the potential to inhibit proteolysis, emphasizing the interplay of different metabolic pathways.
- Reduction of Oxygen in Culture Media:
- Oxygen levels in the culture media can be effectively reduced by various agents such as glucose, thioglycollate, glutathione from cooked meat pieces, cysteine, and ascorbic acid. These reducing agents contribute to creating an anaerobic environment, supporting the growth of anaerobic microorganisms.
References
- http://www.oxoid.com/UK/blue/prod_detail/prod_detail.asp?pr=CM0081&c=UK&lang=EN
- https://himedialabs.com/TD/M149.pdf
- https://www.bd.com/europe/regulatory/Assets/IFU/US/L007448(10)(0706).pdf
- https://www.microxpress.in/product.php?type=A&c_id=9&id=322
- http://www.tulipgroup.com/MicroExpress/Accumix/PackInsert/DehydratedCultureMedia/CFiles/Cooked_Meat_Medium_Robertson_Cooked_Meat_Medium.pdf
- https://universe84a.com/robertsons-cooked-meat-rcm-medium/