Quantitative Buffy Coat Test Overview(QBC Test)
Two different methods of blood tests, thin and thick microscopy, are considered to be the “gold standard” for the diagnosis of malaria. A Quantitative Buffy Coat(QBC) is an additional quick and direct test to make the identification of malaria. It is based on acridine-orange staining of blood samples from peripheral circulation centrifuged in microhematocrit tubes (QBC) and the examination under ultraviolet lighting source (fluorescence microscope).
The color acridine orange is found in the nucleic acid-containing cells and the associated fluorescence can be observed in blue-violet light under microscopes.
Based on the claims of the maker, QBC Malaria Test is 5.5 to 7percent higher effective as Giemsa thin films. It is able to detect as little as one parasite per milliliter of blood, and diagnose before thick films in 47 percent cases of parasitemia low (<10 parasites per milliliter) cases.
QBC is recognized as an effective method for diagnosing blood parasites which cause malaria, filariasis, or visceral leishmaniasis.
Principle of Quantitative Buffy Coat Test (QBC Test)
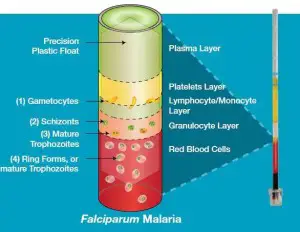
- The principle behind QBC method is based on the fact that upon centrifugation at a high rate the blood is separated into buffy coat, plasma and a packed cells of red. The float is buoyed by blood cells packed in and is then automatically placed in the layer of buffy coat.
- The blood cells that make up the layer of buffy coat separate in proportion to their density and form visible bands. There are platelets on top, lymphocytes and monocytes inside the middle layer, and Granulocytes in the bottom.
- Acridine orange is the reason the malarial parasite stain is in green (nucleus) in addition to an orange (cytoplasm). The tube is studied within the area between red blood cells as well as granulocytes, and in the granulocytes as well as the mononuclear cell layer, which is where parasites are the most prevalent.
- Acridine orange binds with the deoxyribonucleic acid and ribonucleic acid. The malaria parasite is bound by acridine orange in the nucleus as well as the cytoplasm.
- It emits red and green fluorescence when stimulated with blue light (at the wavelength of 460 nuclei) allowing the detection and analysis of the morphology of the parasite using fluorescence microscopy.
- The parasite’s nuclei emit greenish yellowish fluorescence while the cytoplasm displays vibrant red fluorescence.
- RBCs aren’t colored by dye and therefore they are not visible under the fluorescent lighting (dark background) but the brightly glowing parasites can be easily identified.
- The outline of the parasites stained are well preserved, and their general shape is similar to those of the specimens stained with The Giemsa stain.
Sample collection: The blood sample may be taken via the capillary finger-prick or via phlebotomy inside an ethylenediamine tetraacetate (EDTA) vial.
What is QBC tube?
The QBC glass capillary tube (Becton Dickinson) is 75 millimeters long and 1.677 millimeters in diameter. The tubes are coated internally with EDTA and Heparin at the filling end and with acridine-orange stain and potassium oxalate on the other end.
Procedure of Quantitative Buffy Coat Test(QBC Test)
- Take blood samples ( 55ul) into the QBC tube through capillary action.
- Rotate the tubes over for 10 seconds in order to dissolve the residues that are in the blood.
- Place a cylindrical insert or a plastic floating inside an acridine orange-coated capillary tube.
- In the centrifuge, you can run the tubes with 12,000 grams for 5 mins.
After gentrification, blood components and malaria parasites are separated according to density and are concentrated into distinct layers.
Note: The float due to its density is placed over the red cells packed by centrifuge. It covers 90% of the surface area of the tube. This aids in expanding the cells that are segregated by centrifugal force. It is surrounded by three distinct and now quantifiable levels of the buffy coat. - Inject the centrifuged QBC Malaria test into the Paraviewer. Place the tube in a position where the closure extends over the depressed portion in the holders.
- The area that surrounds the float, just below the coat of buffy was studied under the oil-immersion method. Cells within the layer could be easily observed using microscopy. They also showed malaria parasites stained in green (DNA) or the orange (RNA) in blue and violet light.
- The entire tube was scrutinized in detail in a manner that was able to move off from the buffy coating to an erythrocyte layer.
- Each tube was scrutinized until parasites were found or for five minutes maximum.
Results/Analysis of Quantitative Buffy Coat Test(QBC Test)
If a sample is contaminated with P. falciparum malaria parasites:
- Gametocytes that have a shape of a crescent (1) are found at the intersection of the monocyte/lymphocyte and platelet layers.
- A small amount of (2) the schizonts and (3) adult trophozoites might be found inside the granulocyte layer.
- A ring-shaped (4) Immature trophozoites is seen all over the layer of red blood cells with the highest concentration at the border of the layer of granulocytes.
Other species of parasites, such as P. Vivax, may also be concentrated. During centrifugation, they show different characteristics during centrifugation.
Advantages of Quantitative Buffy Coat Test(QBC Test)
- Highly sensitive test for malaria parasite.
- The speed of the QBC method (15 minutes) in the detection of malarial parasites is a certain advantage for laboratories that test a large amount of samples.
- It can easily detects low levels of parasitaemia (2 parasites/ml).
- There isn’t any loss of parasites through the procedure.
- Another advantage of QBC is its ease of interpretation and it is technically easy to perform.
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.