What is Presence/Absence Test?
- The availability of potable water for bathing, drinking, and cooking is essential for modern society.
- Diseases such as diarrhoea, typhoid, cholera, etc., can be transferred by feces-contaminated water and other sources.
- Different methodologies for bacteriological water testing have been developed.
- Weiss and Hunter presented a streamlined method for bacteriological testing of treated water. Later, the PA (Presence Absence) test was created as a streamlined version of the test based on the idea that 100 ml samples of treated water should not include coliforms and other bacterial markers of pollution.
- Clark et al. analysed other facets of the PA exam (3). The inclusion of PA Broth as a provisional standard in the Standard Methods for the Examination of Water and Wastewater is based on the assumption that a 100 ml sample of drinking water should not contain any coliform.
- The Presence Absence (PA) test for the coliform group is a straightforward adaptation of the multiple-tube methods that offers a qualitative estimate of coliforms.
- This test is intended for normal water distribution system or water treatment plant samples.
- When the PA test is positive, coliform densities in repeated samples can be calculated quantitatively to indicate the level of contamination.
- PA test enhances coliform detection in samples containing several species that could cause coliform overgrowth and detection difficulties.
Principle of Presence/Absence Test
- The medium contains peptic digest of animal tissue, tryptose, and beef extract, which provide coliforms with nitrogenous growth factors and trace components.
- The fermentable carbohydrate and energy source for bacterial metabolism is lactose.
- Phosphates have a buffering effect, but sodium lauryl sulphate inhibits numerous species besides coliforms.
- The pH indicator is bromocresol purple, which turns yellow in acidic pH conditions. The majority of lactose-fermenting coliforms use lactose to produce acid.
- The pH indicator (Bromocresol purple) detects this acidity by changing colour from purple to yellow at acidic pH.
- When analysing 100 ml samples, a triple-concentration medium is utilised.
- The PA test is solely indicative of the presence of coliforms. These results must be confirmed using a medium such as Brilliant Green Bile Broth (M121).
Objective of Presence/Absence Test
- To detect the presence of coliforms in treated waters.
PA Broth Preparation
Composition
| Ingredients | Gms / Litre |
| Peptic digest of animal tissue | 5.000 |
| Tryptose | 9.830 |
| Beef extract | 3.000 |
| Lactose | 7.460 |
| Sodium chloride | 2.460 |
| Dipotassium phosphate | 1.350 |
| Monopotassium phosphate | 1.350 |
| Sodium lauryl sulphate | 0.050 |
| Bromo cresol purple | 0.0085 |
Final pH ( at 25°C) 6.8±0.2
Preparation of PA Broth
- To create a 3X concentrate (the most common preparation), dissolve 91.5 grammes of medium in one litre of deionized water.
- To dissolve entirely, bring to a boil while stirring.
- Dispense into suitable containers and sterilise at 121 degrees Celsius for twelve minutes.
Procedure of PA Test
Sample collection
- Utilize a sterile glass or plastic container, such as a Whirl-Pak® bag, for sodium thiosulfate that has been sterilised. If the sample does not contain any residual disinfection, sodium thiosulfate is not required.
- Open the sample containers just prior to collection, and close them soon afterward. Do not place the cap or lid down. Do not touch the container’s lip or inside surface. Before using, do not rinse the containers.
- To obtain a sample of drinkable water from a faucet, spigot, hydrant, or pump, allow the water to flow at a moderate pace for two to three minutes. Remove all filters and aerators. Do not use leaky or swivelling faucets or spigots.
- To obtain a sample of non-potable water from a river, lake, or reservoir, remove the cap while submerged. Alternately, remove the cap and push the container’s mouth down into the water to prevent surface scum from accumulating. Completely fill the container with water. Place the container’s mouth in the current.
- Collect at least 100 mL of sample and maintain at least 2.5 cm (1 inch) of air space in the container.
- Inscribe the sample details on the container and initiate the analysis immediately.
- If the analysis cannot begin immediately, maintain the sample at or below 10 °C (50 °F) for up to eight hours. Do not freeze the sample.
P/A procedure
Method 1: P/A procedure with bottles
- In a sterile container, collect 100 mL of samples. Use aseptic approach to prevent contamination. Note: If the sample has been disinfected, use a dechlorinating agent-containing container.
- The sample should be added to the fill line of a P/A bottle. Note: Non-disinfected samples can be added directly from the faucet or spout.
- 24 hours should be spent incubating the sample at 35 0.5 °C (95 0.9 °F).
- After 24 hours, watch for a shift in colour. If there is no change in colour, the sample should be incubated for a further 24 hours.
- If after 48 hours there is no change in colour, the test result is negative.
- If a colour shift is observed, the test result is presumptively positive.
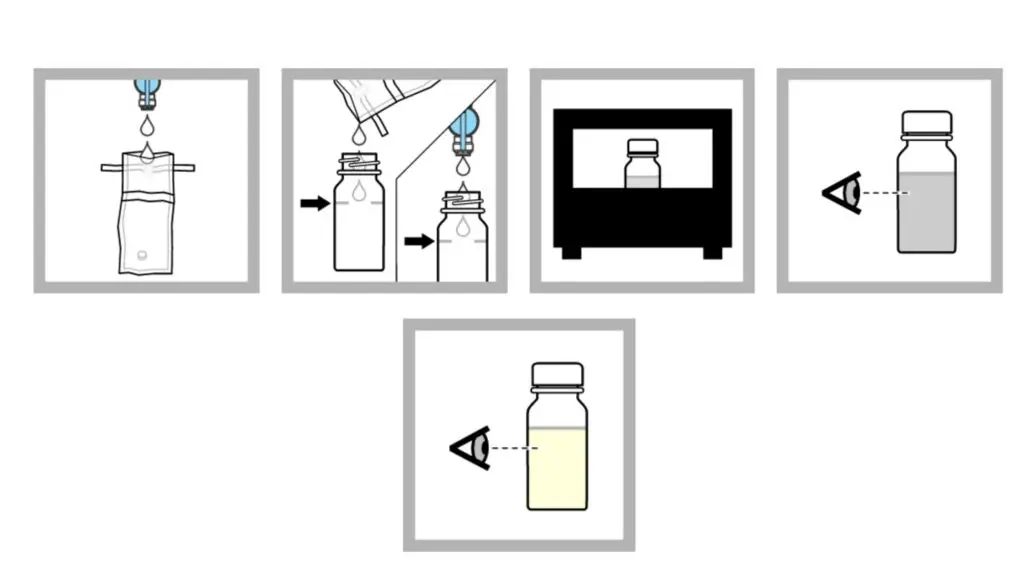
Method 2: P/A procedure with ampules
- Collect one hundred millilitres of sample in a sterile container. Use aseptic approach to prevent contamination. Note: If the sample has been disinfected, use a dechlorinating agent-containing container.
- The sample should be added to the fill line of a sterile sampling vial. Note: Non-disinfected samples can be added directly from the faucet or spout.
- Use an ampule breaker to open a broth ampule aseptically. Add the ampule’s contents to the bottle.
- 24 hours should be spent incubating the sample at 35 0.5 °C (95 0.9 °F).
- After 24 hours, watch for a shift in colour. If there is no change in colour, the sample should be incubated for a further 24 hours. If after 48 hours there is no change in colour, the test result is negative.
- If a colour shift is observed, the test result is presumptively positive.
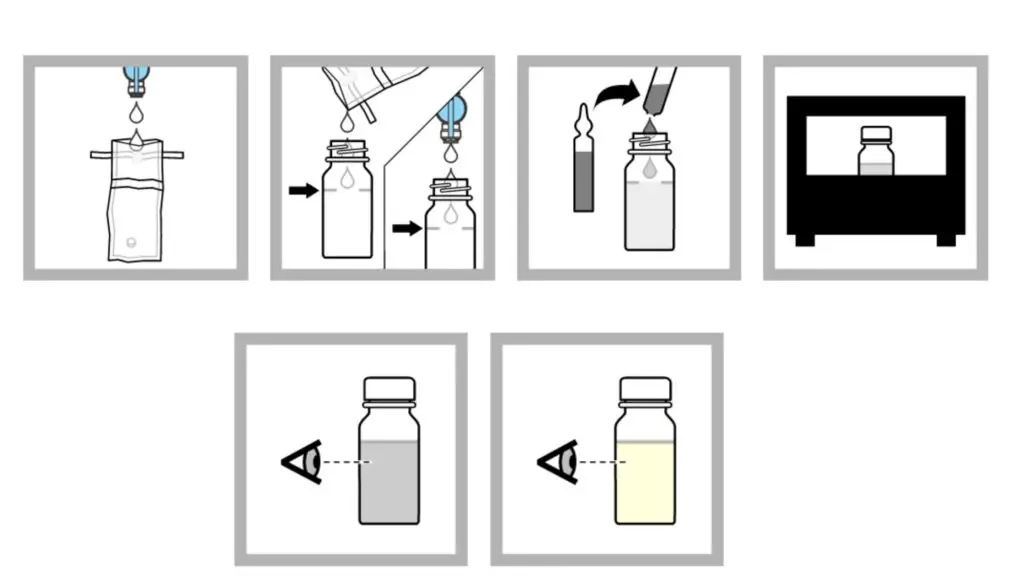
Confirmation procedure
Method 1: Confirmation procedure of P/A Test
All positive test results must be confirmed with a confirmation medium. Confirmation media inhibits the development of non-target organisms while promoting the growth of target organisms. Use the UV confirmation technique if the medium for the presumptive procedure contains MUG.
- Employing a sterile inoculating loop, transfer a portion of the sample culture from the presumptive method to a confirmation medium.
- Incubate the infected confirmation media for the appropriate time and temperature.
- After the incubation period, examine the inner tube for gas production and turbidity. A positive result indicates the presence of coliform bacteria in the sample.
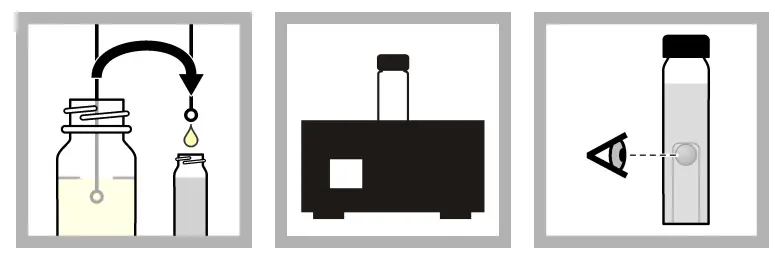
Method 2: UV confirmation procedure
When the nutritional media contains MUG, confirm the presence of E. coli using a long-wave (e.g., 365 nm) UV lamp. The sample will glow if it contains E. coli. There is no need for an additional confirmation procedure.
The sample container may emit a little fluorescent glow. Utilize an E. coli Fluorescence Standard to aid in fluorescence detection. Compare the sample’s fluorescence to that of the standard.
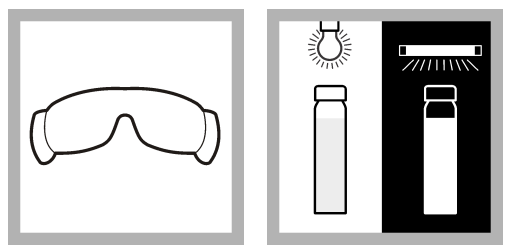
- Put on UV protective glasses.
- The incubated sample containing MUG broth should be illuminated with a long-wave UV lamp. If the sample fluoresces, the presence of E. coli bacteria is confirmed.
Controls for coliform bacteria tests
- Positive and negative controls verify that the test yields a positive result when coliform bacteria are present in the sample and a negative result when they are absent.
- It is recommended to use Pseudomonas aeruginosa as a negative control and Escherichia coli as a positive control.