What is Pollen?
- In the captivating world of plant reproduction, a seemingly unremarkable yet crucial player takes center stage: pollen. This minuscule grain, comprising just a few cells, plays a vital role in the perpetuation of plant life. To the naked eye, pollen appears as a delicate yellowish dust, its ethereal presence often carried away by the wind or the gentle touch of insects.
- The journey of pollen begins within the anthers of plant flowers. Nestled within these anthers are sacs, known as microsporangia, responsible for pollen formation. The intricate process of developing these plant anthers involves the growth and differentiation of specific tissues, ultimately giving rise to the pollen sacs. Inside these sacs, a series of cell divisions, known as meiosis, occurs, resulting in the formation of clusters called quartets.
- As the quartets undergo further divisions, they transform into the precious pollen grains, which serve as the male gametophyte in seed plants, responsible for reproduction. The destiny of these pollen grains diverges depending on the type of plant they belong to – they are either dispersed by the wind or hitch a ride on insects to reach their destination, the receptive stigma.
- In plants that rely on the wind for pollination, they are fittingly called anemophilous. These pollen grains are released into the air and carried away by the slightest breeze, seeking out the elusive receptive stigmas of other plants to initiate fertilization. On the other hand, some plants have evolved to attract insects for pollination, earning the title of entomophilous. These plants entice insects with their vibrant colors, enticing scents, and nectar, effectively enlisting them as couriers to deliver their pollen cargo to the awaiting stigmas of other flowers.
- Once a pollen grain finds its destination, the receptive stigma, it springs into action. Absorbing water and becoming activated, the gametophyte within the grain begins to develop a tiny tube that stretches toward the ovule of the flower. This microscopic tube acts as a conduit, facilitating the transportation of male gamete cells to the ovule for the critical process of fertilization.
- In this enchanting tale of plant life, the unassuming pollen emerges as a silent hero, ensuring the continuation of countless plant species. Its journey from the anther to the stigma, driven by the whims of the wind or the aid of insects, showcases the ingenuity of nature’s designs. So, the next time you see the gentle drift of pollen in the air or spot a bee dusted in yellow, take a moment to appreciate the remarkable role played by these tiny marvels in the grand symphony of life.
Requirements for Pollen Microscopy
- Microscope: The cornerstone of pollen microscopy is, of course, the microscope itself. A good quality light microscope with variable magnification capabilities is essential. The microscope allows researchers to observe pollen grains in fine detail, revealing their unique shapes, surface patterns, and intriguing structures.
- Glass Slides and Coverslips: To prepare pollen samples for examination, glass slides and coverslips are vital components. A small drop of liquid containing the pollen grains is placed on a glass slide, and a coverslip is gently lowered over it. This creates a thin, even layer of pollen grains, enabling easy visualization under the microscope.
- Microscopy Stains: In some cases, staining agents are used to enhance the visibility of pollen grains. Stains can highlight specific features, making it easier to distinguish between different types of pollen. Common stains used in pollen microscopy include iodine, acetocarmine, and safranin.
- Needle (or wire loop): Handling individual pollen grains requires precision and delicacy. A needle or a wire loop is employed to carefully manipulate pollen samples without damaging them. This tool is especially useful when transferring a single grain to a slide or when isolating specific pollen grains for detailed examination.
- Pipette Tips: When working with liquid samples, such as preparing pollen suspensions or dilutions, pipette tips come into play. They allow for accurate and controlled dispensing of liquids, ensuring consistent sample preparation.
- Tweezers: Tweezers are indispensable for handling larger pollen samples or for removing unwanted debris that might interfere with the examination. They offer a steady grip and allow researchers to maneuver pollen grains with precision.
- Camera Attachments: To document and share the mesmerizing beauty of pollen grains, camera attachments are often used with microscopes. These attachments enable researchers to capture high-resolution images and even record videos, aiding in scientific documentation and communication.
- Polarizing Filters: In specialized studies, polarizing filters can be incorporated into the microscope setup. These filters enhance the contrast and reveal unique optical properties of pollen grains, particularly useful in identifying different types of pollen.
Sample Collection for Pollen Microscopy
- Selecting Healthy Flowers: The first step in sample collection for pollen microscopy involves selecting flowers with healthy anthers. Anthers are the male reproductive organs of flowers where pollen grains are produced. The selection process may require inspecting multiple flowers to identify those with anthers bearing abundant and robust pollen content. Healthy anthers are crucial as they ensure the integrity and viability of the pollen grains, providing accurate insights into the plant’s reproductive system.
- Extracting Pollen Grains: Once the ideal flowers with healthy anthers are identified, the process of pollen extraction begins. Using a needle or a laboratory wire loop, researchers carefully and gently scrape the pollen from the anthers. This delicate maneuver ensures that the pollen grains remain intact and undamaged during the collection process.
- Storing the Pollen: After extraction, the collected pollen is carefully transferred to a suitable storage container, such as a pipette tip or a micro-tube. These containers provide a safe and controlled environment for preserving the pollen until it is ready for examination. The use of specialized laboratory equipment, like pipette tips, facilitates accurate and efficient sample handling.
- Immediate Use vs. Preservation: Depending on the research objectives and timelines, researchers may choose to use the collected pollen immediately for microscopy or preserve it for future investigations. When immediate use is planned, the pollen can be directly prepared for examination on microscope slides. However, if the analysis is scheduled for a later date, proper preservation is essential to maintain the pollen’s viability and integrity.
- Drying and Freezing: For long-term storage, the collected pollen should be dried and stored frozen. Drying helps remove excess moisture that could lead to degradation, while freezing ensures the preservation of pollen in a dormant state. These combined measures protect the pollen from enzymatic and biochemical processes that could compromise its quality over time.
Observation of Pollen Under Stereo Microscope
Requirements
- Stereo Microscope: At the heart of this captivating exploration lies the stereo microscope. Unlike traditional compound microscopes, a stereo microscope provides a three-dimensional view of the specimen, offering depth perception and enhancing the overall visual experience. This is crucial for observing pollen grains, which often possess intricate structures and surface features that require precise examination from different angles.
- 50 Percent Glycerine: Glycerine serves as a vital mounting medium for pollen observation. A 50 percent glycerine solution is commonly used to create a viscous and transparent environment that allows pollen grains to be suspended and observed without being crushed or damaged. This medium preserves the natural shape and characteristics of pollen, providing an accurate representation of their true forms.
- Microscope Slide: A clean and appropriately sized microscope slide is a fundamental requirement for preparing pollen samples for observation. A drop of the glycerine solution, containing the pollen grains, is carefully placed on the slide to create a thin, even layer for examination.
- Alcohol: Before mounting the pollen on the microscope slide, it is essential to clean the slide thoroughly to ensure a clear and undistorted view. Alcohol is often used to clean the slide and remove any impurities or residues that could interfere with the observation process.
Procedure for Observation of Pollen Under Stereo Microscope
Observing pollen grains under a stereo microscope is a captivating journey into the hidden world of botanical wonders. With its ability to provide a three-dimensional view, the stereo microscope reveals the intricacies and unique features of these tiny particles, offering invaluable insights into plant reproduction and pollination mechanisms. To embark on this fascinating exploration, a simple yet precise procedure is followed, allowing researchers and students to uncover the mysteries held within each pollen grain.
- Preparation of Pollen Samples:
- a. Collecting Pollen: Begin by collecting healthy anthers from flowers known to contain abundant pollen. The anthers serve as the source of pollen grains for observation. It is advisable to choose anthers from multiple flowers to ensure a diverse and representative sample.
- b. Washing Pollen Grains: To prepare the pollen for observation, it is important to clean the grains, removing any surface impurities and oily substances. Using a small amount of alcohol, gently wash the pollen grains to ensure they are free from any contaminants. This step enhances the visibility and clarity of the pollen under the microscope.
- Sample Mounting:
- a. Microscope Slide Preparation: Take a clean microscope slide and place a drop of the washed pollen sample on it. Additionally, keep a separate drop of unwashed pollen for comparison during the observation process.
- b. Addition of 50 Percent Glycerine: Next, add a drop of 50 percent glycerine to the washed pollen sample on the microscope slide. Glycerine acts as a mounting medium, providing a viscous and transparent environment that preserves the integrity and natural shape of the pollen grains.
- Observation under the Stereo Microscope:
- a. Positioning the Slide: Carefully position the prepared slide, containing the washed pollen sample and glycerine, under the stereo microscope. Adjust the focus and lighting to obtain a clear view of the pollen grains.
- b. Exploring Untreated Pollen: For comparison and to observe the differences, place the microscope slide with the unwashed pollen (untreated sample) on the stage of the stereo microscope. Observe how the untreated grains appear and compare them to the treated ones.
- Analyzing Pollen Grains: Under the stereo microscope, the pollen grains will appear as grossly shaped, irregular structures or particles. Each type of pollen will exhibit distinct characteristics based on its species. Treated pollen grains, free from surface impurities, will display enhanced contrast and clarity compared to untreated ones.
Observation of Pollen Under Compound Microscope
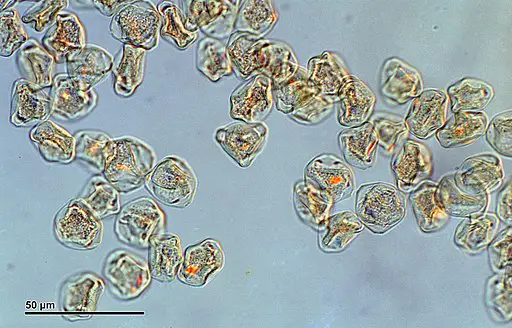
Glycerol Jelly Method for Observation of Pollen Under Compound Microscope
One of the frequently employed methods for mounting pollen is the glycerol jelly technique. This method utilizes a glycerol jelly solution comprising 10 grams of gelatin, 35ml of distilled water, and 30 ml of glycerol.
For achieving optimal results, it is recommended that students utilize freshly collected pollen.
Requirements
- Freshly Collected Pollen Sample: The first and most crucial requirement for the glycerol jelly method is a freshly collected pollen sample. Pollen is a highly sensitive biological material, and its viability and structural integrity decrease over time. To ensure accurate analysis, it is essential to collect the pollen sample as close to the time of observation as possible.
- Compound Microscope: A compound microscope is a fundamental tool in pollen analysis. It enables researchers to observe pollen grains at high magnifications, allowing for detailed examination of their morphology, size, and other characteristics. The microscope should be equipped with appropriate objectives (e.g., 10x, 40x, and 100x) for comprehensive analysis.
- Glycerine Jelly: Glycerine jelly serves as the mounting medium for the pollen grains on the glass slide. The glycerol helps preserve the structure and appearance of the pollen grains during observation, making it easier to identify and differentiate between various species.
- Warm Water: Before starting the glycerol jelly method, warm water is required to soften the glycerine jelly. By warming the jelly, it becomes easier to handle and apply on the glass slide, ensuring a uniform and smooth mounting surface for the pollen grains.
- IPA Solution (Isopropyl Alcohol Solution): The IPA solution, commonly known as isopropyl alcohol, is used to clean the glass slide and cover slips before mounting the pollen sample. It helps remove any dust or debris that may interfere with the observation process, ensuring a clear and accurate analysis.
- Glass Slide: A clean and high-quality glass slide is necessary for mounting the pollen sample. It provides a flat surface for spreading the glycerine jelly and evenly distributing the pollen grains for observation.
- Microscope Cover Slips: Microscope cover slips are essential for covering the mounted pollen sample on the glass slide. They protect the pollen grains from external contaminants and prevent the glycerol from evaporating, ensuring the preservation of the sample during prolonged observations.
- Distilled Water: Distilled water is used in various steps of the glycerol jelly method, such as cleaning the glass slide and preparing the glycerine jelly solution. Distilled water is free from impurities, preventing any contamination that could affect the integrity of the pollen sample.
Procedure
- Take a small dish and place a small amount of the sample (pollen) into it.
- Add a few drops of IPA solution to the dish and let it sit for approximately 10 minutes. The isopropyl alcohol will remove the oily layer from the surface of the pollen grains.
- If the pollen is still attached to the anthers, the IPA solution will help isolate the pollen grains by separating them from the anthers.
- Warm the glycerine until it melts, and keep it warm during the procedure.
- Using a dropper, take 1 or 2 drops of the IPA and pollen mixture and place them at the center of a clean glass slide. Prepare two slides in this manner.
- Put the slide with the mixture on a hotplate and allow it to dry for about 2 minutes.
- Take a microscope cover slip and add one drop of the melted glycerine jelly onto it.
- Gently lower the cover slip, at an angle, onto one of the prepared slides. This step is crucial to remove air bubbles and ensure the jelly comes into full contact with the pollen.
- Allow the slide (with jelly) to stand for approximately 5 minutes on the hotplate. This allows the glycerine to penetrate the pollen grains.
- Once the jelly has been given enough time to penetrate the pollen, remove the slide from the hotplate and let it cool in a cool area until the jelly sets.
- Optionally, if a more extended preservation period is desired, add a small amount of nail polish to seal the edges of the cover slip. This will allow the preparation to be stored and used for several months.
- Finally, view both prepared slides under a microscope to examine the pollen grains.
Note: Ensure all the equipment used is clean and sterile to obtain accurate and reliable results.
Observation
During the observation of the Glycerol Jelly Method under the microscope, distinct differences in the appearance of the stained and unstained slides become apparent, providing valuable insights into the characteristics of the pollen grains.
Upon viewing the stained slide, one can observe a significant improvement in clarity due to enhanced contrast. The pollen grains manifest as minuscule ovoid particles, exhibiting intriguing scaly surfaces or delicate ornamentations. The staining process with glycerine jelly enables a more detailed examination of the grains, allowing for a better understanding of their morphology and structure.
Conversely, the unstained slide presents a more translucent and transparent view, lacking the pronounced contrast observed in the stained slide. As a result, the grain surfaces are not as distinctly visible. However, it is important to note that the appearance of the pollen grains on the unstained slide can also be influenced by the specific plant species from which the pollen was collected. Different plant species may have varying characteristics and structures, leading to diverse visual appearances on the unstained slide.
In conclusion, the Glycerol Jelly Method proves to be a valuable technique for enhancing the clarity and contrast of pollen grains when viewed under a microscope. The stained slide offers a clearer and more detailed perspective, revealing the fascinating features of the grains, while the unstained slide may still provide valuable information, especially considering the impact of plant diversity on their appearance. This method serves as a crucial tool for pollen analysis and aids researchers in gaining deeper insights into the world of botanical morphology.
Wet Mount Technique for Observation of Pollen Under Compound Microscope
Requirements for Wet Mount Technique
- Glycerol: Glycerol serves as a fundamental element in the Wet Mount Technique. It is a viscous and clear liquid that acts as a mounting medium. Glycerol is particularly favored for its refractive index, which closely matches that of glass, allowing for minimal distortion and improved visualization of the specimen under the microscope. Its use ensures that the specimen remains relatively unchanged, preserving its natural characteristics and structure during observation.
- Glass slide: A clean and transparent glass slide forms the foundation of the Wet Mount Technique. It provides a flat and stable platform on which the specimen can be arranged and observed. The glass slide must be free from any impurities or residue to prevent interference with the specimen’s clarity during microscopy. Its smooth surface allows for easy movement of the specimen and ensures that it remains secure during the observation process.
- Cover slip: The cover slip, also known as a cover glass, is a thin and transparent piece of glass that plays a pivotal role in the Wet Mount Technique. It acts as a protective cover for the specimen, preventing it from being crushed or contaminated during observation. The cover slip also assists in spreading the specimen uniformly across the glass slide, allowing for a consistent view of the sample under the microscope. It is vital to use cover slips of appropriate size, ensuring they fully cover the specimen without extending beyond the edges of the glass slide.
- Dropper: A dropper, often in the form of a plastic or glass pipette with a narrow tip, is a necessary tool for the precise application of liquids. In the Wet Mount Technique, the dropper is used to add the glycerol (or other chosen mounting medium) to the specimen on the glass slide. Its controlled and measured delivery of liquids ensures that the mounting medium spreads evenly, avoiding excessive overflow and potential damage to the specimen.
Procedure
- Begin by placing a clean glass slide on a flat surface in preparation for the wet mount technique.
- Using a dropper, add precisely two drops of glycerol onto the center of the glass slide. Glycerol serves as the mounting medium in this method, facilitating better visualization of the specimen.
- Obtain a small sample of pollen, which can be collected by gently tapping the anther of the flower. Carefully transfer the pollen grains to the glycerol on the slide. Ensure that the pollen is evenly dispersed and not clumped together.
- To prevent the formation of air bubbles, gently position a transparent cover slip at an angle over the sample on the glass slide. Lower it slowly to cover the pollen, allowing the glycerol to spread uniformly across the specimen.
- For extended preservation and to avoid contamination, you may apply a small amount of nail polish along the edges of the cover slip. This creates a secure seal, protecting the specimen from external elements.
- Once the wet mount slide is fully prepared, carefully place it on the microscope stage for observation.
- If enhanced clarity and contrast are desired, staining the sample can be performed. For this purpose, toluidine blue or acetocarmine can be used. Simply add a few drops of the staining agent to the sample before covering it with the cover slip.
- Finally, adjust the microscope settings, including focus and illumination, to obtain a clear and detailed view of the pollen specimen.
The Wet Mount Technique offers a simple yet effective means of observing live or preserved specimens under the microscope. By following this step-by-step procedure and using glycerol as the mounting medium, researchers can gain valuable insights into the microscopic world of pollen and other fascinating samples. Staining with toluidine blue or acetocarmine further enhances the visibility of the specimen, providing a deeper understanding of its structure and characteristics.
Dry Mounting Technique
The Dry Mounting Technique is a straightforward and uncomplicated method for preparing specimens for microscopic observation. Follow these simple steps to effectively perform the procedure:
- Prepare the Glass Slide: Start by placing a clean and dry glass slide on a flat surface. Ensure that the slide is free from any dust, residues, or contaminants that could interfere with the observation.
- Position the Sample: Carefully place the sample, in this case, the pollen, onto the center of the glass slide. Arrange the pollen grains in a single layer to avoid overlap and ensure a clear view under the microscope.
- Add the Cover Slip: Once the pollen sample is appropriately positioned on the slide, gently lower a transparent cover slip over the sample. Place the cover slip at an angle to allow any trapped air to escape, preventing the formation of air bubbles that could obstruct the observation.
- Observation under the Microscope: With the sample securely mounted between the glass slide and cover slip, the slide is now ready for microscopic examination. Place the prepared slide on the microscope stage and adjust the focus and illumination settings as needed to obtain a clear and detailed view of the pollen specimen.
The Dry Mounting Technique offers a quick and uncomplicated way to observe various specimens under the microscope. By following these three simple steps, researchers and students can explore the fascinating microcosm of pollen and other microscopic entities with ease. Remember that the Dry Mounting Technique is suitable for specimens that do not require additional preparation or treatment, making it an ideal choice for obtaining immediate observations.
Observation Pollen Tubes under Microscope
Pollen tubes are crucial structures that grow down the style and deliver gametes to the ovary for fertilization. Farmer’s solution and sodium hydroxide can be used to see pollen tubes under a microscope.
Requirements for Observation of Pollen Tubes under Microscope
To successfully observe pollen tubes under a microscope, the following essential requirements and reagents are necessary for proper preparation and examination:
- Mimulus ringens Flowers (with healthy anthers): A suitable source of Mimulus ringens flowers with healthy anthers is required. Healthy anthers ensure the presence of viable pollen, which is crucial for studying pollen tube growth.
- 70% Ethyl Alcohol (Ethanol): Ethyl alcohol serves as a fixative to preserve the structure and integrity of the pollen tubes. It helps in preventing degradation and maintaining the original morphology of the pollen tubes during the staining process.
- Farmer’s Solution: Farmer’s solution is an essential fixative used specifically for the study of pollen tubes. It aids in stabilizing and preserving the pollen tubes, allowing for subsequent staining and observation under the microscope.
- 0.4M Sodium Sulphite Solution: Sodium sulphite solution is used to remove excess Farmer’s solution after fixation. This step helps in preventing any interference during the staining process and enhances the clarity of the observation.
- 1 Molar Sodium Hydroxide: Sodium hydroxide is utilized to promote pollen tube germination. It creates an alkaline environment that stimulates the growth of pollen tubes from the pollen grains.
- 8 M NaOH or 10 M NaOH: These concentrated solutions of sodium hydroxide can be diluted to the required concentration (1 Molar) for promoting pollen tube growth. Care must be taken while handling these strong bases.
- Aniline Blue, Aniline Blue with Fluorescent Brightener, or Acetocarmine combined with Acidified Aniline Blue: These staining agents are employed to visualize and highlight the pollen tubes. They aid in enhancing the contrast and visibility of the pollen tubes under the microscope, making it easier to study their structure and growth patterns.
Procedure
To observe pollen tubes under a microscope, follow these step-by-step procedures, which involve fixation, staining, and decolorization techniques:
- Prepare the Sample: Using a pair of tweezers, carefully separate the pistils from the rest of the flower’s components. The pistils contain the female reproductive structures, including the ovary and stigma, where pollen tubes are present.
- Fixation: Place the separated pistils in a suitable fixing agent, such as Farmer’s solution, ethyl alcohol, or formalin-acetic acid-alcohol. Allow the sample to remain in the fixing agent for approximately 36 hours to preserve the pollen tubes’ structure and prevent degradation.
- Transfer to Ethanol: After fixation, transfer the sample to 70 percent ethanol. Ethanol serves to remove excess fixative and prepare the sample for subsequent processing.
- Distilled Water Softening: Immerse the sample in distilled water for around 10 minutes to prepare it for softening. This step helps to condition the sample for optimal visualization of the pollen tubes.
- Softening Solution: Place the sample in a softening solution of either 8 M NaOH or 10 M NaOH for approximately 10 hours. The softening process promotes the emergence and visibility of pollen tubes from the pollen grains.
- Washing: After softening, remove the sample from the softening solution and thoroughly wash it with distilled water. Ensure that the ovary is removed during the washing process.
- Staining: Choose one of the staining agents mentioned in the previous content (aniline blue, aniline blue with fluorescent brightener, or acetocarmine combined with acidified aniline blue) to stain the sample. The staining process enhances the contrast and visibility of the pollen tubes.
- Decolorization: Submerge the stained sample in a 0.1% solution of aniline blue in potassium phosphate for approximately 2 hours. Perform this step in a dark room to optimize decolorization. The decolorization process removes excess stain, ensuring a clearer view of the pollen tubes.
- Mounting: Remove the sample from the decolorizer and place it on a clean microscope slide. Add a drop of the decolorizer to the slide and gently position a cover slip over the sample.
- Microscopic Observation: The prepared slide is now ready for observation. Place it under the microscope and adjust the focus and illumination to observe and study the structure and growth patterns of the pollen tubes.
Observation
Upon observation of pollen tubes under the microscope, a remarkable and delicate sight unfolds. The pollen tubes exhibit a unique appearance, resembling slender and fragile tubes, akin to flexible straws. Their elongated structures are visible with precision and clarity, allowing for a closer examination of their intriguing morphology.
As one delves into the microscopic world, the delicate nature of the pollen tubes becomes apparent. The tubes extend with purpose, exhibiting remarkable flexibility and adaptability in their growth. The microscope reveals a fascinating array of these slender structures, each representing the growth and developmental journey of a pollen grain.
The tubes’ texture appears smooth and translucent, showcasing the intricate passage through which the pollen grains navigate in their quest for fertilization. Their size and shape may vary, depending on the plant species and the specific stage of growth under examination. Some may be relatively straight, while others might display gentle curves and bends, adapting to the intricacies of their surrounding environment.
As the microscope’s lens captures this mesmerizing sight, researchers and botanists gain valuable insights into the reproductive biology of plants. The observation of pollen tubes plays a crucial role in understanding the mechanisms of pollination, fertilization, and successful seed production in diverse plant species.
The fragile tubes, like flexible straws, encapsulate the incredible journey of life and reproduction in the plant kingdom. Their growth and navigation towards the ovary present a testament to nature’s intricate design and the beauty of botanical processes.
In conclusion, the observation of pollen tubes under the microscope unveils a captivating display of fragile and flexible tubes. This microscopic exploration grants a deeper appreciation for the complexities of plant reproduction and highlights the significance of pollen tubes in the perpetuation of life in the botanical realm.
Observation of Pollen Under Electron Microscopy
Acetolysis is one of the most common procedures for preparing pollen for electron microscopy. However, it has been demonstrated that it causes distortions, which encouraged the development of a new and improved approach. Aerosol-OT and amyl acetate are two of the best and most current approaches. Acetolysis is the process of breaking down substances (organic compounds) using acetic acid.

Requirements
The observation of pollen under electron microscopy involves a set of specific requirements and techniques to achieve high-resolution imaging. To prepare and study pollen samples using electron microscopy, the following essential components are needed:
- Aerosol-OT: Aerosol-OT (AOT) is a surfactant commonly used in preparing pollen samples for electron microscopy. It assists in dispersing the pollen grains evenly, enabling better visualization and imaging under the microscope.
- Flower Sample: Obtain the flower sample from the plant of interest. The flower should be at the appropriate stage of development to ensure the presence of mature and viable pollen grains for analysis.
- Water: Water serves as a crucial component for sample preparation and is used to create various solutions required during the process.
- Acetone and Ethanol: Acetone and ethanol are utilized as dehydrating agents to remove moisture from the pollen samples. Dehydration is a critical step to preserve the ultrastructure of the pollen during electron microscopy.
- Amyl Acetate: Amyl acetate is a solvent used in sample preparation. It is effective in dissolving lipids and other non-polar substances that may interfere with electron microscopy imaging.
- Scanning Electron Microscope (SEM): The scanning electron microscope is the primary tool used for imaging pollen samples at high magnifications and resolutions. It allows researchers to observe the surface topography and three-dimensional structure of the pollen grains.
- Pipettes: Precise pipettes are essential for accurate and controlled transfer of liquids during the sample preparation process.
- 15 ml Centrifuge Tubes: Centrifuge tubes are used to store and handle the prepared pollen samples during various stages of the electron microscopy preparation.
Procedure
The observation of pollen under electron microscopy involves a detailed and precise preparation process to obtain high-quality images. Follow these step-by-step procedures for successful electron microscopy imaging of pollen grains:
- Softening Anthers: Carefully remove the anthers from the flower and immerse them into a 3 percent Aerosol-OT solution for softening. This step allows for easier extraction of the pollen from the anthers.
- Extracting Pollen: Gently remove the pollen from the softened anthers and place them into a 15 ml centrifuge tube. Ensure that the pollen is collected with care to preserve its integrity.
- Rehydration: Keep the pollen sample in the Aerosol-OT solution for approximately 5 days for rehydration. This process revitalizes the pollen grains, preparing them for subsequent processing.
- Water Wash: Using a pipette, drain out the Aerosol-OT solution from the centrifuge tube and add water to the tube. Allow the pollen to soak in the water for about 10 minutes to remove excess Aerosol-OT.
- Acetone/Water Mixture: After the water wash, drain out the water and add an acetone/water mixture (1:1) to the tube. Let the pollen sample sit in this mixture for about an hour. This step aids in dehydration and prepares the pollen for further processing.
- Centrifuge and Sonicate: Lightly centrifuge the tube to concentrate the pollen grains at the bottom. Next, place the tube in an ultrasonic bath for a minute to sonicate the grains gently, facilitating thorough cleaning.
- Mixture Solution: Drain out the acetone/water mixture and replace it with a fresh batch. Allow the mixture to stand for about 1 hour to clean any residual material from the surface of the pollen grains.
- Ethanol Dehydration: Replace the acetone/water mixture with distilled water and allow the pollen to stand for 10 minutes. Remove the distilled water and add 50 percent ethanol, letting it stand for an hour. Subsequently, replace the 50 percent ethanol with 70 percent ethanol and allow it to stand for an hour.
- Ethanol Gradation: For dehydration, replace the 70 percent ethanol with 90 percent ethanol for an hour. Then, replace the 90 percent ethanol with 100 percent ethanol and repeat this step twice. During the second round with 100 percent ethanol, allow it to stand for about 4 hours to ensure effective dehydration.
- Amyl Acetate Preparation: Drain out the ethanol and add amyl acetate to the tube for about an hour. This step is essential to prepare the pollen for critical drying.
- Modified BEEM Capsule: Remove the pollen grains from the tube and transfer them to a modified BEEM capsule.
- Mounting the Grains: Cover the ends of the capsule and press the cylinder cap. Carefully remove excess amyl acetate while ensuring the pollen grains are retained.
- Transfer to Baskets: Using a pipette, transfer the pollen grains into baskets suitable for electron microscopy.
- Critical Point Drier: Place the baskets in the critical point drier for optimal drying of the pollen grains.
- Shadowing with Gold: Before viewing, shadow the pollen grains with gold. This coating enhances electron microscopy imaging and provides a more detailed examination of the pollen structure.
Observation
Upon observation of pollen under the Scanning Electron Microscope (SEM), a fascinating world of intricate structures unfolds. The grains present themselves as unique and diverse entities, each bearing distinct features depending on the type of pollen being viewed. The SEM offers unparalleled resolution, revealing detailed characteristics of the pollen grains.
One striking observation is the varying inflation levels of the grains. Some pollen grains appear inflated, while others display a deflated appearance. The shapes of the grains also exhibit remarkable diversity, ranging from spherical to elliptical or even irregular forms. These shape variations reflect the diversity of plant species and their reproductive strategies.
The surfaces of the pollen grains showcase intriguing attributes. Some grains present rough surfaces with cleavages, creating textured patterns that contribute to their distinctive appearance. The presence of these surface features can vary significantly, depending on the specific type of pollen being observed.
Under compound microscopes or electron microscopes, the pollen grains may exhibit diverse ornamentations. These ornamentations can take on different forms and patterns, adding another layer of complexity to their structure. Irregularly distributed across the surface of the grains, these ornamentations contribute to the overall aesthetics of the pollen.
The distribution of these ornamentations is not uniform. While some grains may display ornamentations across their entire surface, others may only feature them at specific regions, such as the polar ends or in particular sections of the grain. This non-uniform distribution adds to the uniqueness of each pollen grain, reflecting the intricate and specialized adaptations developed by plants for efficient pollination.
In conclusion, the observation of pollen under the SEM or compound microscopes unveils a captivating display of diversity and complexity. The grains, with their inflated or deflated structures and various shapes, exhibit distinct surface features, including ornamentations irregularly distributed across their surfaces. This wealth of characteristics provides valuable insights into the world of pollination, plant reproduction, and the intricate relationships between plants and their pollinators. The observation of pollen under these powerful microscopes contributes to our understanding of the ecological and biological significance of these tiny yet indispensable agents in the world of botany.
FAQ
What is pollen?
Pollen is a fine, powdery substance produced by the male reproductive organs of flowering plants. It contains the male gametes (sperm cells) and is essential for plant reproduction.
Why is pollen observed under the microscope?
Observing pollen under the microscope allows scientists and researchers to study its structure, morphology, and various features, providing valuable insights into plant reproductive processes and pollination mechanisms.
How is pollen prepared for microscopy?
Pollen is typically prepared for microscopy by using various techniques such as wet mount or staining methods. Fixatives, solvents, and mounting media are used to preserve and enhance the visibility of pollen grains.
What are the common staining agents used for pollen observation?
Common staining agents for pollen observation include aniline blue, acetocarmine, and toluidine blue. These stains help highlight specific structures and enhance contrast for better visualization.
What does pollen look like under the microscope?
When observed under the microscope, pollen grains appear as various shapes, such as spherical, elliptical, or irregular. They may exhibit surface ornamentations, which differ depending on the plant species.
What is the significance of pollen observation in plant taxonomy?
Pollen observation is crucial in plant taxonomy as it helps identify and classify different plant species based on the unique characteristics of their pollen grains.
How do pollen tubes appear under the microscope?
Pollen tubes, which facilitate the transfer of male gametes to the female reproductive organs, appear as delicate, elongated structures resembling flexible straws when viewed under the microscope.
Can electron microscopy be used to study pollen?
Yes, electron microscopy provides high-resolution imaging of pollen, allowing for detailed examination of their ultrastructure and surface features.
What can the study of pollen reveal about plant evolution?
The study of pollen can provide insights into plant evolution, migration, and adaptation, as pollen characteristics can change over time, reflecting evolutionary trends and relationships between plant species.
How is pollen analysis used in environmental studies?
Pollen analysis, also known as palynology, is utilized in environmental studies to reconstruct past climates, vegetation changes, and human impacts on ecosystems based on the presence and abundance of different pollen types in sedimentary deposits.