What is Plasmolysis?
- Plasmolysis, a term rooted in the Latin word “plasma” signifying ‘matrix’ and the Greek term “lysis” denoting ‘loosening’, refers to the phenomenon where plant cells undergo contraction due to water loss.
- This process is instigated when cells are subjected to a hypertonic environment, leading to the efflux of water from the cell. Conversely, when a cell is placed in a hypotonic solution, it experiences a diminished external osmotic pressure, resulting in deplasmolysis or cytolysis, where there is a net influx of water into the cell.
- Observing these two processes can provide insights into the tonicity of the cellular environment and the rate at which solute molecules traverse the cellular membrane.
- In a more detailed context, eukaryotic plant cells, distinct from their animal counterparts, possess specialized cellular organelles. A prominent feature of plant cells is their robust cell wall, which provides structural integrity and prevents deformation.
- Within the cell, the cytoplasm, plasma membrane, and other organelles collaboratively ensure the cell’s functionality. The vacuole, a membrane-bound organelle, serves as a reservoir for water within the plant cell.
- However, under specific conditions, plant cells may either be deprived of adequate water or undergo substantial water loss. This leads to pronounced contraction of the plant cell, a state referred to as plasmolysis.
- It is noteworthy that plasmolysis is not a frequent natural occurrence but is primarily observed under extreme conditions. In experimental settings, it can be induced by immersing cells in concentrated saline or sucrose solutions, facilitating exosmosis.
- Commonly, plant cells from Elodea or onion epidermis with colored cell sap are utilized to visually demonstrate this process. The staining of plant cells with methylene blue can further enhance the visibility of plasmolysis.
- In summation, plasmolysis is a critical concept in plant cell biology, highlighting the cell’s response to osmotic challenges and offering a deeper understanding of cellular water dynamics and membrane permeability.
Definition of Plasmolysis
Plasmolysis is the process in which plant cells contract or shrink due to the loss of water when exposed to a hypertonic solution.
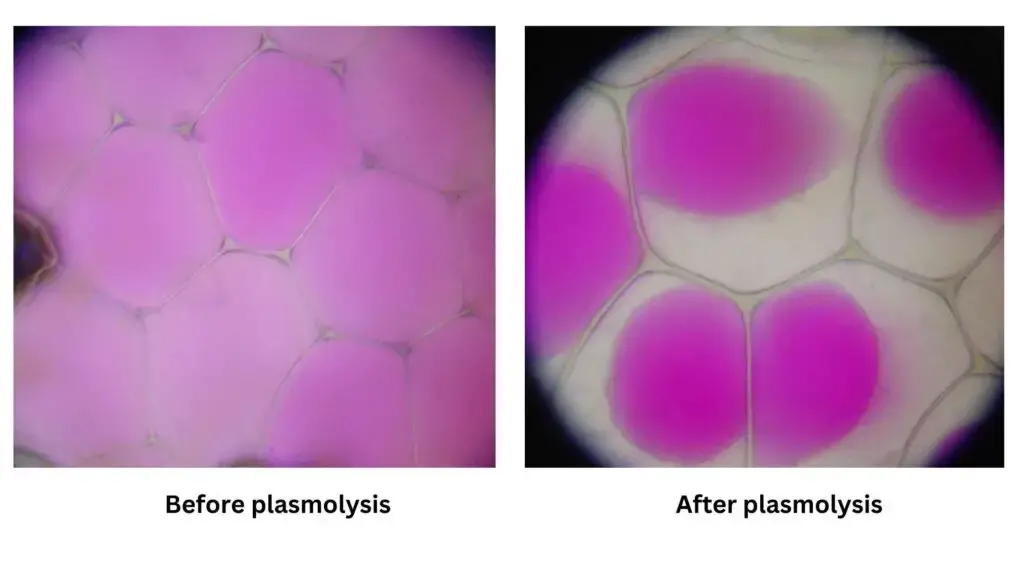
Stages of Plasmolysis
Plasmolysis is a complex process that occurs in plant cells when exposed to hypertonic solutions, leading to water loss and cellular contraction. This process can be delineated into three methodical stages:
- Incipient Plasmolysis: Initiated by the efflux of water from the cell, this preliminary stage witnesses a reduction in the cell’s volume. Concurrently, the cell wall, which provides structural support, becomes more discernible.
- Evident Plasmolysis: Progressing to this stage, the cell’s contraction reaches its maximum limit. As a result, the cytoplasm, which houses the cell’s components, begins to detach from the cell wall, adopting a more spherical configuration.
- Final Plasmolysis: Culminating the process, this stage sees the cytoplasm fully disengaging from the cell wall. The cytoplasm then centralizes, positioning itself at the heart of the cell.
In essence, plasmolysis is a sequential event that underscores the dynamic nature of plant cells in response to osmotic challenges, emphasizing the intricate balance between cellular structure and function.
Plasmolysis and Osmosis
Plasmolysis and osmosis are intricately linked processes that play pivotal roles in the regulation of water movement in cells. Understanding their interrelation provides insights into cellular dynamics and the maintenance of cellular homeostasis.
Osmosis: At its core, osmosis is a specialized form of diffusion, specifically concerning the movement of water molecules across a semipermeable membrane, such as the plasma membrane of a cell. The direction of this water movement is governed by the concentration gradient of solutes in the solution surrounding the cell. Solutions can be categorized based on their solute concentration relative to the cell’s internal environment:
- Hypertonic Solution: Characterized by a higher solute concentration outside the cell, water molecules move out of the cell to equilibrate solute concentrations. This outflow of water can lead to plasmolysis, where the cell contracts due to water loss.
- Hypotonic Solution: Here, the external solute concentration is lower than inside the cell, prompting water to rush into the cell. For plant cells, this influx results in turgidity, where cells become swollen and exert turgor pressure against one another, providing structural support to the plant.
- Isotonic Solution: In this scenario, the solute concentrations inside and outside the cell are equivalent, ensuring no net movement of water across the membrane.
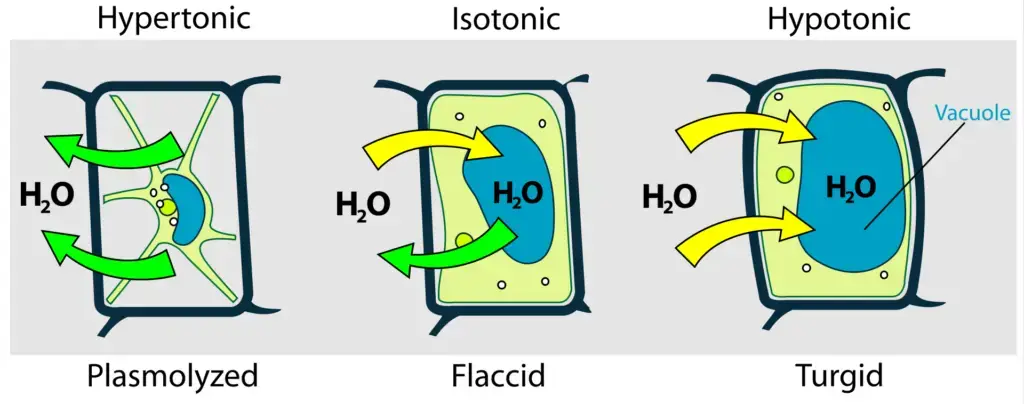
Plasmolysis: Directly influenced by osmosis, plasmolysis is the phenomenon where plant cells lose water and undergo contraction when placed in a hypertonic solution. This water loss is a direct consequence of osmotic forces driving water out of the cell to balance solute concentrations.
Plant cells exhibit optimal functionality in hypotonic environments. When engorged with water, they become turgid, exerting turgor pressure against neighboring cells, forming the plant’s foundational support. The presence of a rigid cell wall in plant cells prevents them from bursting, even when filled with water. In contrast, animal cells, devoid of a cell wall, are susceptible to lysis (bursting) when exposed to hypotonic solutions due to excessive water intake. Hence, they thrive best in isotonic conditions.
In summary, the interplay between plasmolysis and osmosis underscores the significance of osmoregulation in cells, emphasizing the delicate balance required to maintain cellular integrity and function.
Passage of Water through Cell Membrane
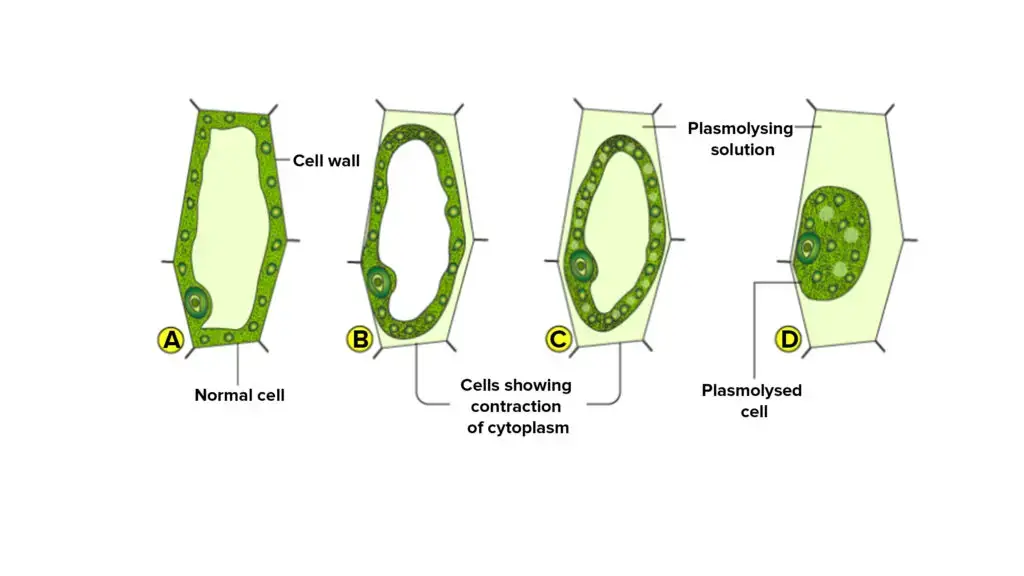
The passage of water through the cell membrane is a fundamental process that underpins various cellular activities and responses. This movement is particularly evident during the phenomenon of plasmolysis in plant cells.
- Mechanism of Water Movement: In the context of plasmolysis, the cell membrane delineates the cell’s internal environment from its external surroundings, specifically the cell wall. This demarcation facilitates the movement of water molecules, ions, and select particles across the membrane. The inherent permeability of the cell membrane allows water molecules to traverse it with ease, ensuring that cells maintain their necessary hydration levels.
- Osmosis and Plasmolysis: Osmosis, the diffusion of water across a selectively permeable membrane, plays a pivotal role in the movement of water through the cell membrane. When plant cells are immersed in a concentrated salt solution, as in a laboratory setting, they experience an osmotic drive that prompts water to flow out from the cell sap into the surrounding medium. This outflow results from the higher solute concentration outside the cell, compelling water to move out to equilibrate concentrations. As a consequence, the cell’s protoplasm detaches from the cell wall and assumes a spherical configuration.
- Experimental Observations: Certain plants, such as Rheo, Elodea, and onion epidermal cells, are commonly employed in laboratory experiments to demonstrate plasmolysis. The choice of these specific plants is attributed to their colored sap, which enhances visibility under a microscope. This characteristic allows for a clear observation of the water movement and the resultant changes in cell morphology during plasmolysis.
In summation, the movement of water through the cell membrane is a dynamic process, intricately linked to the cell’s osmotic environment. Plasmolysis serves as a tangible demonstration of this movement, emphasizing the cell membrane’s role in regulating water flow and maintaining cellular homeostasis.
Types of Plasmolysis
Plasmolysis, a phenomenon observed in plant cells when subjected to hypertonic solutions, can be classified into two distinct types based on the eventual configuration of the cytoplasm:
- Concave Plasmolysis: Characterized by the retraction of both the protoplasm and the cell membrane from the cell wall due to water loss, concave plasmolysis is a reversible process. The affected cell can regain its original state when reintroduced to a hypotonic solution, which facilitates water re-entry into the cell, restoring its turgidity.
- Convex Plasmolysis: In this type, the cell undergoes a more severe form of water loss, leading to the complete detachment of the protoplasm and cell membrane from the cell wall. As a consequence, the cell wall collapses, resulting in the irreversible destruction of the cell. This form of plasmolysis is indicative of extreme dehydration and is more intricate than its concave counterpart.
In essence, the types of plasmolysis underscore the varying degrees of cellular response to osmotic challenges, highlighting the delicate balance that plant cells must maintain to ensure their viability and structural integrity.
Causes of Plasmolysis
Plasmolysis arises primarily due to osmotic imbalances within the cellular environment. Delving deeper into the causes of plasmolysis:
- Exosmosis: Plasmolysis is instigated by the process of exosmosis, where water molecules migrate from regions of lower solute concentration to areas of higher solute concentration across the cell membrane. This movement is driven by the inherent desire of molecules to achieve equilibrium in their surroundings.
- Hypertonic Solutions: When plant cells are immersed in hypertonic solutions, solutions with elevated solute concentrations compared to the cell’s internal environment, they experience water loss. This results in the cell’s contraction, a direct manifestation of plasmolysis.
- Turgor and Osmotic Pressure: In contrast, when plant cells are placed in hypotonic solutions, they absorb water through osmosis. This influx of water increases the internal pressure, causing the protoplasm to press against the cell walls, a state termed as turgor. Turgor is vital for plants as it provides structural rigidity, enabling them to maintain an upright posture. At full turgor, plant cells are so engorged that they exert pressure against one another, preventing further water intake. This equilibrium is crucial for plant health and stability. However, when the surrounding environment lacks sufficient air spaces, osmotic pressure intensifies, gradually diminishing turgor.
In essence, plasmolysis is a consequence of osmotic imbalances, reflecting the cell’s adaptive responses to external solute concentrations. It underscores the significance of osmoregulation in maintaining cellular integrity and overall plant health.
Importance of Plasmolysis
Plasmolysis plays a pivotal role in the physiological responses of plant cells when exposed to hypertonic environments. Delving into its significance:
- Cellular Response to Hypertonic Conditions: Plasmolysis is a cellular mechanism employed by plant cells when confronted with hypertonic solutions. In such conditions, the protoplasm retracts and detaches from the cell wall, creating a space that becomes occupied by solutes.
- Water Regulation: The detachment of the protoplasm from the cell wall serves as a signal for the plant to address the osmotic imbalance. This prompts the plant to absorb water from its roots, thereby aiding in restoring the cell’s turgidity.
- Stomatal Mechanism: Concurrently, the plant also activates its stomatal machinery to minimize water loss. This dual-action ensures that the plant maintains its water balance, highlighting the adaptive nature of plasmolysis.
- Protective Mechanism: While plasmolysis can be viewed as a protective response, it’s crucial to note its limitations. If the process progresses to an extreme, leading to cytorrhysis, the cell may suffer irreversible damage, compromising its structural integrity and potentially leading to cell death.
In essence, plasmolysis underscores the resilience and adaptability of plant cells, enabling them to navigate and respond to osmotic challenges, ensuring their survival and overall health.
Deplasmolysis
Deplasmolysis is a vital cellular process that occurs when a previously plasmolysed cell is exposed to a hypotonic environment. Delving into its intricacies:
- Cellular Response to Hypotonic Conditions: When a cell, previously subjected to plasmolysis, is placed in a hypotonic solution, it experiences a higher concentration of water outside its membrane. This gradient drives water molecules to move into the cell.
- Cellular Turgidity: As water continues to flow into the cell, it swells, regaining its turgidity. This restoration of the cell’s original state is termed as Deplasmolysis.
- Isotonic Equilibrium: In scenarios where cells are immersed in isotonic solutions, where the solute concentration is identical both inside and outside the cell, there is a balanced movement of water. In such conditions, water molecules move in and out of the cell at an equal rate, maintaining a state of equilibrium. Consequently, the cells remain in a flaccid state, neither turgid nor plasmolysed.
In essence, deplasmolysis underscores the ability of plant cells to recover from a plasmolysed state, highlighting the dynamic nature of cellular osmoregulation. This process ensures that cells can adapt and respond to varying osmotic conditions, maintaining their structural integrity and functionality.
Examples of Plasmolysis
Plasmolysis is a fundamental cellular process, predominantly observed in plant cells, where the cell’s protoplasm shrinks and detaches from the cell wall due to water loss. This phenomenon can be induced under hypertonic conditions. Here are some illustrative examples of plasmolysis in various contexts:
- Blood Cells in Hypertonic Solutions: When blood cells are immersed in hypertonic solutions, they undergo shrinkage. The water from inside the cells moves out to balance the solute concentration, leading to the cells’ contraction.
- Vegetables in Saline Environments: Vegetables, when exposed to hypertonic conditions, such as in a saline solution, exhibit shrinkage due to the outflow of water from their cells.
- Coastal Flooding and Salt Deposition: During severe coastal flooding events, ocean water inundates terrestrial regions, depositing salt onto the land. This salt creates a hypertonic environment for the local vegetation, leading to water loss from plant cells and, in many cases, plant death.
- Weed Control Using Weedicides: In agricultural practices, chemical weedicides are employed to eliminate unwanted weeds. These chemicals induce plasmolysis in the weed cells, effectively killing them.
- Food Preservation: In the food industry, high concentrations of salt or sugar are added to certain food items, such as jams, jellies, and pickles, to act as preservatives. This hypertonic environment causes water to move out of any microbial cells present, inhibiting their growth and thus preserving the food.
In essence, plasmolysis is a critical process with implications in various fields, from agriculture and food preservation to medical science. Its understanding aids in harnessing its effects for beneficial purposes and mitigating its adverse impacts in specific scenarios.
Plasmolysis vs. Cytolysis
Plasmolysis and cytolysis are two distinct cellular processes influenced by osmotic pressures, each resulting from different environmental conditions. Here’s a comparative analysis of the two:
Plasmolysis:
- Definition: Plasmolysis is the process where the cell’s protoplasm shrinks and detaches from the cell wall due to water loss.
- Cause: It occurs when a cell is exposed to a hypertonic solution, leading to the outflow of water from the cell to balance the solute concentration outside.
- Result: The cell shrinks, and in plant cells, the protoplasm detaches from the cell wall.
- Protection in Plants: Plant cells have a rigid cell wall that prevents them from undergoing complete shrinkage. Instead, they experience a reduction in turgor pressure.
Cytolysis:
- Definition: Cytolysis refers to the bursting or rupture of a cell due to an excessive influx of water.
- Cause: This phenomenon arises when a cell is placed in a hypotonic solution, causing water to diffuse into the cell.
- Result: When the volume of water inside the cell surpasses the cell membrane’s capacity, the cell bursts, a process evident in red blood cells.
- Protection in Plants: Plant cells are safeguarded against cytolysis due to the presence of a cell wall, which provides structural support and prevents the cell from bursting even when it swells. Instead, the cell becomes turgid due to increased turgor pressure.
In essence, while plasmolysis results from water loss in a hypertonic environment, cytolysis is the outcome of water gain in a hypotonic setting. Cytolysis can be viewed as the reverse of plasmolysis, with each process representing the cell’s response to different osmotic challenges.
| Aspect | Plasmolysis | Cytolysis |
|---|---|---|
| Definition | Process where the cell’s protoplasm shrinks and detaches from the cell wall. | Bursting or rupture of a cell due to an excessive influx of water. |
| Cause | Exposure to a hypertonic solution leading to water outflow from the cell. | Exposure to a hypotonic solution causing water to diffuse into the cell. |
| Result | Cell shrinks; protoplasm detaches from the cell wall in plant cells. | Cell bursts; evident in red blood cells. |
| Protection in Plants | Reduction in turgor pressure but cell wall prevents complete shrinkage. | Cell wall provides structural support, preventing bursting; cell becomes turgid with increased turgor pressure. |
Plasmolysis vs. Turgidity
Plasmolysis:
- Cells lose water through exosmosis, leading to a net water efflux.
- The cell experiences shrinkage as the protoplasm and the plasma membrane pull away from the cell wall.
- This phenomenon occurs when the cell is in a hypertonic solution.
- There’s a drop in turgor pressure.
- As a result, plants tend to wilt.
Turgidity:
- Cells gain water through endosmosis, leading to a net water influx.
- The cell swells as the protoplasm and the plasma membrane press against the cell wall.
- This phenomenon occurs when the cell is in a hypotonic solution.
- There’s a rise in turgor pressure.
- As a result, plants exhibit rigidity and stand upright.
| Aspect | Plasmolysis | Turgidity |
|---|---|---|
| Water Movement | Cells lose water by exosmosis (i.e. net water efflux). | Cells gain water by endosmosis (i.e. net water influx). |
| Cell State | The cell shrinks as the protoplasm and the plasma membrane pull away from the cell wall. | The cell swells as the protoplasm and the plasma membrane press against the cell wall. |
| Solution Type | Occurs when the cell is in a hypertonic solution. | Occurs when the cell is in a hypotonic solution. |
| Turgor Pressure | Turgor pressure drops. | Turgor pressure rises. |
| Effect on Plants | Results in the wilting of plants. | Results in plant rigidity and being upright of plants. |
Plasmolysis vs. Flaccidity
Plasmolysis:
- Refers to the shrinking of the protoplasm due to exposure to a hypertonic environment.
- The protoplasm and the plasma membrane pull away from the cell wall.
- Results in the wilting of plants.
- Occurs when the cell is in a hypertonic solution.
- The process can be reversed by placing the cell in a hypotonic solution.
Flaccidity:
- Represents the loss of turgor due to the absence of net water movement between the plant cell and its isotonic surroundings.
- A flaccid cell is neither swollen (turgid) nor shrunk (plasmolyzed).
- Similar to plasmolysis in terms of the outcome, leading to the wilting of plants.
- Occurs when the cell is in an isotonic solution.
- The turgor state of the cell can be restored by changing the surrounding solution to a hypotonic one.
| Aspect | Plasmolysis | Flaccidity |
|---|---|---|
| Definition | Shrinking of the protoplasm due to a hypertonic environment. | Loss of turgor due to no net water movement in an isotonic environment. |
| Cell State | Protoplasm and plasma membrane pull away from the cell wall. | Neither swollen (turgid) nor shrunk (plasmolyzed). |
| Result | Wilting of plants. | Wilting of plants, similar to plasmolysis. |
| Occurrence | In a hypertonic solution. | In an isotonic solution. |
| Reversibility | Can be reversed by placing in a hypotonic solution. | Turgor can be restored by changing to a hypotonic solution. |
Concave Plasmolyses vs. Convex Plasmolyses
| Aspect | Concave Plasmolysis | Convex Plasmolysis |
|---|---|---|
| Definition | A type of plasmolysis where the protoplasm shrinks inwardly with respect to the cell wall. | A type of plasmolysis where the protoplasm shrinks in a manner that it may not be reversed. |
| Appearance | Protoplasm shrinks towards the center of the cell. | Protoplasm shrinks uniformly, detaching from the cell wall. |
| Reversibility | Reversible by deplasmolysis when placed in a hypotonic solution. | Irreversible, even when placed in a hypotonic solution. |
| Natural Response in Plants | Plants regulate stomata and produce water-resistant wax to prevent further water loss. | Typically leads to cell death due to irreversible damage. |
Quiz
What is plasmolysis?
a) Swelling of a cell due to water intake
b) Bursting of a cell due to excessive water intake
c) Shrinking of the protoplasm away from the cell wall due to water loss
d) Movement of water molecules from a region of higher concentration to lower concentration
Which solution causes plasmolysis in plant cells?
a) Hypotonic
b) Isotonic
c) Hypertonic
d) None of the above
What happens to a plant cell when it undergoes plasmolysis?
a) It swells up
b) It remains unchanged
c) It shrinks
d) It bursts
Which of the following is a sign of plasmolysis in plant cells?
a) Turgidity
b) Flaccidity
c) Gap formation between the cell wall and the plasma membrane
d) Cell bursting
Which type of plasmolysis is reversible?
a) Concave plasmolysis
b) Convex plasmolysis
c) Both
d) None
What is the opposite process of plasmolysis?
a) Osmosis
b) Diffusion
c) Deplasmolysis
d) Cytolysis
In which condition does plasmolysis occur?
a) When the cell is in a solution with a higher solute concentration
b) When the cell is in a solution with a lower solute concentration
c) When the cell is in a solution with an equal solute concentration
d) None of the above
What is the role of the cell wall during plasmolysis?
a) It prevents the cell from bursting
b) It aids in the shrinking of the cell
c) It remains unaffected
d) It dissolves in the surrounding solution
Which cells are more prone to plasmolysis?
a) Animal cells
b) Plant cells
c) Fungal cells
d) Bacterial cells
What is the main cause of plasmolysis?
a) Active transport
b) Passive transport
c) Osmotic movement of water out of the cell
d) Endocytosis
FAQ
What is plasmolysis?
Plasmolysis is the process where the protoplasm of a plant cell shrinks away from its cell wall due to the loss of water through osmosis.
Why does plasmolysis occur in plant cells?
Plasmolysis occurs when a plant cell is placed in a hypertonic solution, causing water to move out of the cell, leading to the shrinking of its protoplasm.
Is plasmolysis a reversible process?
Yes, plasmolysis is reversible. When the cell is placed back in a hypotonic or isotonic solution, it can regain its turgor through a process called deplasmolysis.
How is plasmolysis different from cytolysis?
While plasmolysis refers to the shrinking of the protoplasm in plant cells due to water loss, cytolysis refers to the bursting of a cell due to the excessive influx of water.
What are the visual signs of plasmolysis in plant cells?
The main visual sign is the gap that forms between the cell wall and the plasma membrane due to the shrinking of the protoplasm.
How does plasmolysis affect the overall plant?
When many cells in a plant undergo plasmolysis, the plant may wilt or droop due to the loss of turgor pressure in its cells.
What solutions can cause plasmolysis in plant cells?
Hypertonic solutions, which have a higher solute concentration than the cell’s cytoplasm, can cause plasmolysis in plant cells.
Is plasmolysis unique to plant cells?
While plasmolysis is most commonly associated with plant cells due to their rigid cell walls, certain other cells with walls, like some bacteria, can also undergo a similar process.
What role does the cell wall play during plasmolysis?
The cell wall provides structural support and prevents the cell from completely collapsing during plasmolysis. It also prevents the cell from bursting when water re-enters during deplasmolysis.
Why is understanding plasmolysis important in botany and agriculture?
Understanding plasmolysis is crucial as it provides insights into plant cell behavior under stress conditions, like drought or high salinity. This knowledge can help in developing strategies for crop management and improving plant resistance to such stresses.
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.