Parasporal crystal staining is the process used to identify and observe the parasporal crystalline inclusions formed by Bacillus thuringiensis during sporulation. It is mainly used because these protein crystals (also called δ-endotoxins) are the major feature of Bt strains, and it is important to differentiate the crystals from the spores and other cell materials. It is a rapid staining method where protein-rich crystals take up specific dyes and become clearly visible. The reaction is based on the affinity of dyes toward the concentrated protein matrix present in the crystal, and the stained crystals appear either dark blue or pink depending on the method used.
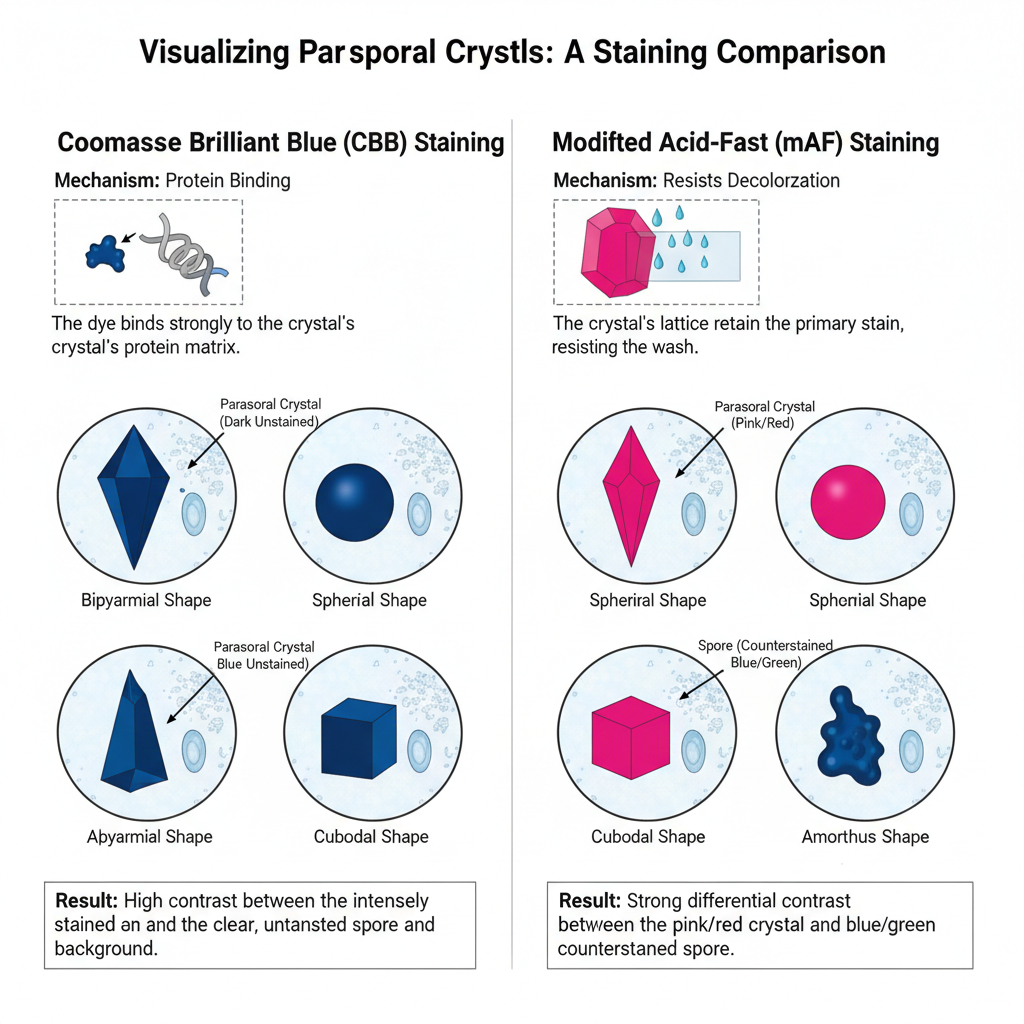
In this staining, two important principles is used. One is the Coomassie Brilliant Blue (CBB) staining where the dye binds with the basic amino acid residues present in the crystal. It is the process in which hydrophobic and ionic interactions help the dye to attach strongly, and the crystals appear deep blue. This is referred to as a quick method because only a few minutes is required for the stain to develop and it gives better visualization of very small parasporal bodies.
Another method is the Modified Acid-Fast (mAF) staining where Carbol Fuchsin is used. In this step the crystal retains the stain even after acid-alcohol treatment because of the compact nature of the protein lattice, so the crystals appear pink to red while other structures are decolorized.
The main purpose of parasporal crystal staining is to confirm the presence and the morphology of the insecticidal crystals such as spherical, cuboidal, bipyramidal or amorphous forms. It is used in selecting new Bt isolates because the type of crystal formed is often related to the Cry or Cyt gene content. It is the process that helps researchers to make initial screening before doing molecular analysis or bioassay studies.
The intensity of the blue color in the CBB stained samples is also used as an indication of total δ-endotoxin yield, so it works as an important, low-cost quality control step in fermentation and production laboratories.
Principle of Parasporal Crystal Stain
The principle of parasporal crystal stain is based on the unique protein composition and dense structure of the parasporal crystalline inclusion produced during sporulation of Bacillus thuringiensis. It is the process where the staining reaction highlights the crystal separately from the spores and other cellular materials. In the Coomassie Brilliant Blue (CBB) method, the dye has a high affinity for protein molecules and it binds strongly with the basic amino acid residues present in the crystal matrix. The interaction is due to hydrophobic forces and ionic bonding, and because the crystal is a highly concentrated protein body, it takes up the stain very deeply. This is referred to as selective staining since the spores and debris remain lightly stained, while the crystal becomes dark blue.
Another principle used is the Modified Acid-Fast (mAF) staining where Carbol Fuchsin is the primary dye. In this step, the dense and compact arrangement of the δ-endotoxin protein gives the crystal a type of functional acid-fastness. It is not true acid-fastness as found in organisms with mycolic acids, but the crystalline lattice helps the dye to remain inside even after acid-alcohol treatment. The reaction is as follows where the crystal appears pink or red while other materials lose the dye easily. These principles help in rapid detection of the parasporal bodies by either targeting the protein-rich nature of the crystal or by using its resistance to decolorization as the differentiating feature.
Purpose
Production of parasporal crystals is a unique ability (among Bacillus species) of Bacillus thuringiensis. This stain is a means of rapid identification of the species.
Requirement for Parasporal Crystal Staining
- Bacterial culture–
- The isolate must be Bacillus thuringiensis (Bt).
- The culture should reach the sporulation stage for formation of parasporal crystalline inclusions (PCIs).
- Sporulation medium (like T3 broth) is used and incubation is usually 48–72 hours.
- Smear preparation–
- A thin smear of the sporulated cells is made on a clean glass slide.
- The smear is allowed to air-dry.
- Fixation is done by brief heat fixation or by methanol fixation.
- Reagents for CBB staining–
- Coomassie Brilliant Blue solution is required.
- Common formulation used is 0.25% CBB with ethanol and acetic acid.
- The fixed smear is flooded with CBB dye for about 3 minutes.
- Water rinse is used to remove background stain.
- Reagents for Modified Acid-Fast staining–
- Kinyoun’s Carbol Fuchsin works as the primary stain.
- Acid-alcohol is needed for decolorization.
- A counterstain such as Malachite Green or Methylene Blue is required.
- Microscope–
- A brightfield light microscope is used for observation.
- Oil immersion objective lens (1000×) is used for viewing the stained crystals.
Procedure of Parasporal Crystal Staining
Steps — Coomassie Brilliant Blue (CBB) method
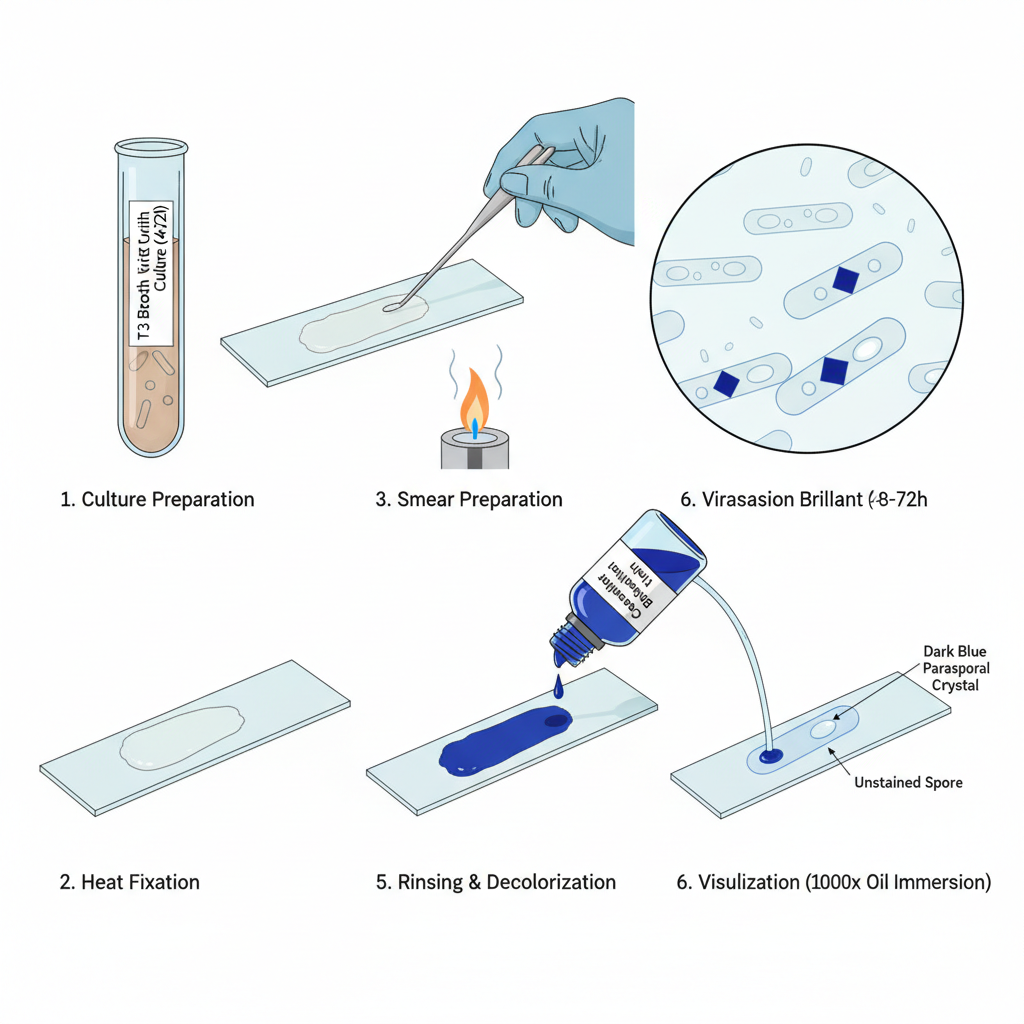
- Culture preparation. It is necessary to use a Bt culture at sporulation stage. Cultures are grown in suitable medium (eg T3 broth) for 48–72 hours to ensure maximal crystal formation.
- Smear preparation. One loopful of sporulated culture is taken. A thin smear is spread evenly on a clean glass slide.
- Fixation. Allow smear to air dry completely. Fix by gentle heat (pass slide briefly through flame) or use slide warmer.
- Primary staining. Flood heat-fixed smear with Coomassie Brilliant Blue solution. Typical composition: 0.25% CBB, 50% ethanol, 7% acetic acid (alternative: 0.1% CBB, 30% methanol, 5% acetic acid). Allow stain to remain for about 3 minutes.
- Rinsing and decolorization. Gently rinse with distilled or running water. Background material and vegetative debris is rapidly decolorized.
- Drying and visualization. Allow slide to air dry. Examine under brightfield microscope with 100X oil immersion objective (1000×).
- Result: Parasporal crystals appear as distinct dark blue inclusions while spores remain largely unstained or clear.
Steps — Modified Acid-Fast (mAF, Kinyoun) method
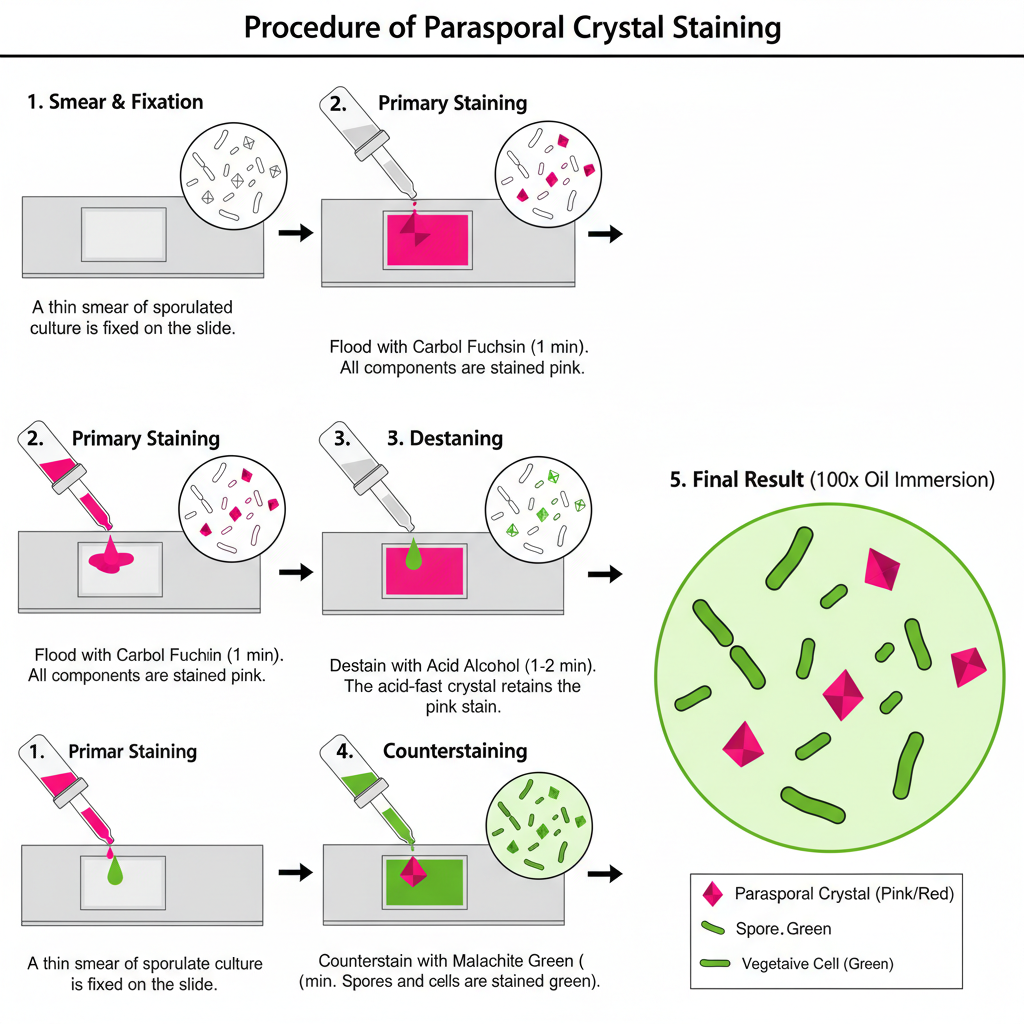
- Culture and smear preparation. Prepare thin smear from sporulated Bt culture on clean slide.
Allow to air dry. - Fixation. Fix smear either by absolute methanol for 30 seconds or by gentle heat fixation.
- Primary staining (Kinyoun Carbol Fuchsin). Flood smear with Kinyoun’s Carbol Fuchsin (no heating of reagents required). Allow stain to sit for 1 minute. Rinse briefly with distilled water and drain.
- Destaining (differential removal). Treat smear with acid alcohol (eg 10 ml H2SO4 + 90 ml absolute ethanol, or 1–3% HCl in 70% ethanol) for 1–2 minutes. Rinse with distilled water and drain. This step removes stain from non-acid-fast material.
- Counterstaining. Flood slide with Malachite Green (3% aqueous) for 2 minutes. Rinse briefly with distilled water and drain.
- Drying and visualization. Dry slide (eg on slide warmer) and examine with 100X oil immersion objective.
- Result: Parasporal crystals retain primary stain and appear pink/red/purple. Background and spores take up counterstain and appear green/blue.
Result
The result of parasporal crystal staining is the clear visualization of the proteinaceous parasporal crystalline inclusion (PCI) that is produced during sporulation. It is observed as a distinct body that is separated from the spore and vegetative cell material, and it is used to confirm the presence and appearance of δ-endotoxins.
In most observations, the stained crystal appears sharply bounded because the dye binds strongly to the protein matrix. The spore and the surrounding cell debris usually remain lightly stained or completely unstained, and this gives a contrast that helps in detecting the crystal body.
Result of Coomassie Brilliant Blue (CBB) Staining
In this staining, the parasporal crystal shows a dark blue appearance.
It is the process where the CBB dye binds to the high protein content inside the crystal and forms a deep blue inclusion under the light microscope.
Some of the main features are–
- The crystal appears as an intense dark blue or deep blue body.
- The background is mostly decolorized after washing, therefore the vegetative cell remnants appear clear.
- In many isolates, the crystal is seen adjacent to the spore and sometimes a blue cap-like structure may be attached with the spore.
- The blue color also indicates the mass of the protein crystal, and it gives an idea of the toxin yield inside the cell
Result of Modified Acid-Fast (mAF) Staining
This process is referred to as a differential stain because the parasporal crystal resists decolorization.
The highly ordered lattice of the crystal retains the primary stain.
Some of the characteristics are–
- The crystal appears pink, red or purple after staining with Carbol Fuchsin.
- The vegetative cell material, spore coat or other debris do not retain the stain and take the counterstain such as methylene blue or malachite green.
- This gives a strong contrast where the crystal is visible as a colored body lying near a blue or green spore.
Result on Crystal Morphology
It is the step where different crystal shapes are observed because staining helps in highlighting the boundaries clearly.
This is important since the morphology has a close relationship with Cry and Cyt gene content.
Some of the common crystal shapes are–
- Bipyramidal crystals, which are historically linked with isolates toxic to Lepidoptera.
- Spherical or ovoid crystals, which are seen in isolates acting against Diptera.
- Cuboidal or rectangular crystals, which are reported in isolates toxic to Coleoptera.
- Amorphous or irregular bodies that show the diversity of toxin gene profiles.
- Rare atypical structures like long spikes or multi-fiber filaments are also detected, which may not be clearly visible under unstained conditions.

Uses of Parasporal Crystal Staining
- It is used for rapid identification of Bacillus thuringiensis strains showing parasporal crystalline inclusions.
- It is the method for confirming the presence of proteinaceous parasporal crystal bodies (PCIs) in environmental isolates.
- It helps in high-throughput screening of large number of colonies in Bt isolation programs.
- It is used as an alternative to phase-contrast microscopy for detecting very small crystals or low number of capped spores.
- It helps in classifying the isolates on the basis of crystal morphology like bipyramidal, spherical, ovoid, cuboid and amorphous shapes.
- It is used for detecting unusual or atypical crystal structures which indicate possible novel toxin genes.
- It gives a presumptive idea about the toxin profile and host range of the isolate before molecular tests.
- It is applied in industrial fermentation units to monitor crystal protein (δ-endotoxin) formation.
- It is used as an initial quality control test where the staining intensity gives a quick idea about total crystal yield.
- It confirms the proteinaceous nature of the inclusion body when CBB stain binds with the hydrophobic and ionic sites of the crystal.
- It is used to assess the physical integrity of the crystal structure by applying modified acid-fast stain that indicate the stability of the crystalline lattice.
Advantages of Parasporal Crystal Staining
- It gives a rapid and high-throughput screening of Bt colonies and the whole preparation process is completed in a short time.
- It provides quick primary confirmation of the parasporal crystalline inclusions (PCIs) which help in rejecting non-producing strains early.
- It shows improved resolution compared to phase-contrast microscopy, allowing very small crystal bodies to be seen clearly.
- It gives high contrast between spores and crystals so both structures is easily differentiated.
- It helps in detecting unusual or atypical structures like very small crystals, capped spores, or spiked spores which may indicate novel toxin profiles.
- It confirms the proteinaceous nature of the inclusion body because the stain binds with high affinity to crystal proteins.
- It gives a visual idea of yield since the dark blue intensity in CBB staining act as a quick proxy of δ-endotoxin production.
- It helps in phenotypic profiling because crystal shape is clearly visible and can be used to predict Cry/Cyt gene content and likely insecticidal activity.
Limitations of Parasporal Crystal Staining
- It is a qualitative method and cannot give a precise quantitative measurement of δ-endotoxin yield.
- It must be followed by advanced analytical methods like CE or LC-MS/MS-MRM for exact identification and quantification of crystal proteins.
- It does not show the specific toxin composition or the exact insecticidal activity of the proteins present.
- It cannot differentiate between insecticidal Cry/Cyt proteins and non-insecticidal proteins like Parasporins.
- It does not detect vegetative phase insecticidal proteins such as Vip or Sip which are secreted into the medium.
- The observed crystal shape only gives a presumptive idea of toxin profile and needs confirmation by genetic or proteomic tests.
- Some crystals may not take up the stain properly and this can create difficulty in visualizing the inclusions.
- Isolates producing very small crystals or very low crystal amounts can give ambiguous or unclear results.
- Modified Acid-Fast staining can sometimes show variable coloration which makes interpretation difficult.
- Ahern, H. (n.d.). Differential Staining Techniques. In Microbiology: A Laboratory Experience. Milne Publishing.
- Bechtel, D. B., & Bulla, L. A., Jr. (1976). Electron Microscope Study of Sporulation and Parasporal Crystal Formation in Bacillus thuringiensis. Journal of Bacteriology, 127(3), 1472–1481. https://doi.org/10.1128/jb.127.3.1472-1481.1976
- Bulla, L. A., Jr, Kramer, K. J., & Davidson, L. I. (1977). Characterization of the entomocidal parasporal crystal of Bacillus thuringiensis. Journal of Bacteriology, 130(1), 375–383. https://doi.org/10.1128/jb.130.1.375-383.1977
- Caballero, J., Jiménez-Moreno, N., Orera, I., Williams, T., Fernández, A. B., Villanueva, M., Ferré, J., Caballero, P., & Ancín-Azpilicueta, C. (2020). Unraveling the Composition of Insecticidal Crystal Proteins in Bacillus thuringiensis: a Proteomics Approach. Applied and Environmental Microbiology, 86(12). https://doi.org/10.1128/AEM.00476-20
- Carbol fuchsin. (n.d.). In Wikipedia.
- Centers for Disease Control and Prevention. (n.d.). DPDx – Laboratory Identification of Parasites of Public Health Concern: Stool Specimens – Staining Procedures.
- Expert Report on Parasporal Crystal Staining Methodologies for Bacillus thuringiensis. (n.d.).
- Georgiou, C. D., Grintzalis, K., Zervoudakis, G., & Papapostolou, I. (2008). Mechanism of Coomassie brilliant blue G-250 binding to proteins: a hydrophobic assay for nanogram quantities of proteins. Analytical and Bioanalytical Chemistry, 391(1), 391–403. https://doi.org/10.1007/s00216-008-1996-x
- Handayani, K., Janah, S. M., Ekowati, C. N., & Kanedi, M. (2023). Study of Morphology and Protein Crystal Character of Bacillus thuringiensis Isolates. IOP Conference Series: Earth and Environmental Science.
- Ibrahim, M. A., Griko, N., Junker, M., & Bulla, L. A. (2010). Bacillus thuringiensis: A genomics and proteomics perspective. Bioengineered Bugs, 1(1), 31–50. https://doi.org/10.4161/bbug.1.1.10519
- Liu, C.-M., & Tzeng, Y.-M. (2001). Quantitative Analysis of Parasporal Crystal Protein from Bacillus thuringiensis by Capillary Electrophoresis. Journal of Food and Drug Analysis, 9(2), Article 2. https://doi.org/10.38212/2224-6614.2796
- Oestergaard, J., Voss, S., Lange, H., Lemke, H., et al. (2007). Quality control of Bacillus thuringiensis ssp. israelensis products based on toxin quantification with monoclonal antibodies. Biocontrol Science and Technology, 17(3), 295–302. https://doi.org/10.1080/09583150701211665
- Optimization of Carbol Fuchsin Kinyoun Staining for Environmental and Clinical Microbiology Samples. (n.d.). ARKdb-chicken Public Database, Roslin Institute.
- Palma, L., Muñoz, D., Berry, C., Murillo, J., & Caballero, P. (2014). Bacillus thuringiensis Toxins: An Overview of Their Biocidal Activity. Toxins, 6(12), 3296–3325. https://doi.org/10.3390/toxins6123296
- Peralta, C., Sauka, D. H., Marozzi, A., Del Valle, E. E., & Palma, L. (2021). Argentinean Bacillus thuringiensis strains exhibiting distinct morphology of their parasporal crystals. Revista Argentina de Microbiología / Argentinean Journal of Microbiology, 53(4), 378–379. https://doi.org/10.1016/j.ram.2020.09.005
- Rampersad, J., & Ammons, D. (2005). A Bacillus thuringiensis isolation method utilizing a novel stain, low selection and high throughput produced atypical results. BMC Microbiology, 5(52). https://doi.org/10.1186/1471-2180-5-52
- Remel. (2013). TB Ziehl-Neelsen Carbolfuchsin (IFU 40102). Thermo Fisher Scientific.
- Schnepf, E., Crickmore, N., Van Rie, J., Lereclus, D., Baum, J., Feitelson, J., Zeigler, D. R., & Dean, D. H. (1998). Bacillus thuringiensis and Its Pesticidal Crystal Proteins. Microbiology and Molecular Biology Reviews, 62(3), 775–806. https://doi.org/110.1128/mmbr.62.3.775-806.1998
- Sharif, F., & Alaeddinoĝlu, N. G. (1988). A rapid and simple method for staining of the crystal protein of Bacillus thuringiensis. Journal of Industrial Microbiology and Biotechnology, 3(4), 227–229. https://doi.org/10.1007/BF01569580
- Xu, C., Wang, B.-C., Yu, Z., & Sun, M. (2014). Structural Insights into Bacillus thuringiensis Cry, Cyt and Parasporin Toxins. Toxins, 6(9), 2732–2770. https://doi.org/10.3390/toxins6092732