Papanicolaou staining (Pap stain) is a multichromatic cytological staining method used for examining exfoliated cells from different body sites. It is the process developed by George Papanicolaou, and it is mainly applied in cervical smears but it is also used for sputum, urine, cerebrospinal fluid and other cytology samples.
It is the universal stain in cytology because it gives a clear distinction between the nucleus and cytoplasm, which is important for identifying abnormal or precancerous cells. The stain uses several dyes in a sequence, and the nuclear part is stained by hematoxylin, producing a blue to black nucleus that helps in observing chromatin pattern and nuclear changes.
The cytoplasmic staining is carried out by Orange G and EA (Eosin Azure) mixtures, and these are responsible for differential staining of keratinized and non-keratinized cells. In this step, keratinized cells appear orange, while metabolically active cells take blue-green to pink shades depending on the cell maturity.
This is referred to as metachromatic staining, and it is controlled by the density and chemical affinity of the cells toward these dyes. The cytoplasm becomes transparent after staining, and this transparency is one of the main features because it allows the nucleus to be observed even when cells are overlapping.
It is the process that requires immediate wet fixation of the smear. The fixation is usually done using 95% ethyl alcohol, because air drying causes distortion of cellular morphology and affects the stain uptake. This process occurs when the specimen is collected and placed directly into the fixative so the structural details are preserved for staining. Even though liquid-based cytology methods are now used to reduce debris and mucus, the Pap stain remains the main diagnostic technique in cytology laboratories. It helps in identifying malignant cells, hormonal changes, and certain infections, and the stain provides consistent results in routine examination of exfoliated cells.
Objectives of Papanicolaou Staining (Pap stain)
- To identify the nuclear component of the cell to assist in the detection of abnormalities in the nuclear structure of cancerous cells.
- For cytoplasm staining, and make it transparent to allow for visual inspection
- To differentiate and distinguish specific cell types like basophils and acidophils.
Principle of Papanicolaou Staining (Pap stain)
The principle of Papanicolaou staining is based on a multichromatic staining reaction where different cell structures take different dyes according to their chemical nature. It is the process in which the nucleus and cytoplasm are separated into basophilic and acidophilic components, and the staining depends on the affinity of these components toward specific dyes. The nuclear staining is carried out by hematoxylin, which binds with the acidic nucleic acids giving a blue to black coloured nucleus that helps in observing chromatin pattern and nuclear margin. After this, a bluing step is used in alkaline medium so that the reddish tone of hematoxylin is converted into deep blue colour.
The next part of the principle depends on cytoplasmic counterstaining using Orange G and EA mixtures. Orange G is acidic and stains keratinized cells in bright orange shades. The EA mixture contains Eosin Y, Light Green and other components along with phosphotungstic acid (PTA). It is the PTA which controls the binding of dyes by preventing Eosin Y from attaching to certain cytoplasmic sites, and because of this, metabolically active cells appear blue-green while the superficial mature cells appear pink. This is referred to as differential cytoplasmic staining and it shows the metabolic maturity of the cells.
It is the process that requires immediate wet fixation in 95% alcohol because air drying causes swelling, distortion and loss of nuclear details. The fixation helps in producing cytoplasmic transparency, allowing the nuclear structure to be clearly seen even when cells are overlapping. The transparency and multicoloured pattern produced by the stain is the key principle behind the diagnostic value of Pap staining.
Composition of the Reagents of Papanicolaou Staining (Pap stain)
The reagents used in Pap staining are prepared in a way that each solution gives a selective colour reaction to different parts of the cells. It is the process in which three main staining solutions are used along with fixatives and bluing agents. These solutions contain five important dyes such as Hematoxylin, Orange G, Eosin Y, Light Green SF and sometimes Bismarck Brown. Each reagent has its own components that help in differential staining of nucleus and cytoplasm.
Nuclear Stain (Hematoxylin Solution)
Hematoxylin is the main nuclear stain used in the first step. Different formulations are used like Harris hematoxylin or Gill’s hematoxylin, but all of them contain hematoxylin dye, a mordant and an oxidizing agent. Harris hematoxylin is prepared by dissolving hematoxylin in alcohol, and aluminum ammonium sulfate (alum) is added as a mordant. The oxidizing agent may be mercuric oxide or sodium iodate to convert hematoxylin into hematein. Glacial acetic acid is also added to increase nuclear staining and to make the solution stable. Gill’s hematoxylin contains hematoxylin dye, ethylene glycol, sodium iodate and aluminum sulfate, and glacial acetic acid is used to control pH.
First Counterstain (Orange G – OG 6)
Orange G solution is used for staining keratinized cells. It is the process in which Orange G dye crystals are dissolved in distilled water and 95% alcohol. The important component is phosphotungstic acid (PTA), and the “6” in OG-6 denotes the amount of PTA used. Some preparations also contain small amounts of acetic acid for better staining.
Second Counterstain (EA Solution)
The EA (Eosin Azure) solution is a polychromatic mixture used for cytoplasmic differentiation. This solution contains Eosin Y which stains mature cytoplasm, Light Green SF which stains metabolically active cytoplasm, and Bismarck Brown Y which is sometimes omitted because it may form precipitates. These dyes are dissolved in alcohol. PTA is added to regulate differential staining by limiting Eosin uptake in certain cells, and glacial acetic acid helps in improving colour intensity. EA 36, EA 50 and EA 65 are common types and the numbers indicate the relative quantity of dyes, especially Light Green.
Ancillary Reagents
95% ethyl alcohol is used as the fixative for wet fixation because it preserves cellular structure. Bluing agents such as Scott’s tap water substitute, dilute ammonium hydroxide or lithium carbonate solution are used to convert the reddish tone of hematoxylin into a blue colour. These agents are alkaline and help in stabilizing the nuclear stain.
Procedure for Papanicolaou Staining (Pap stain)
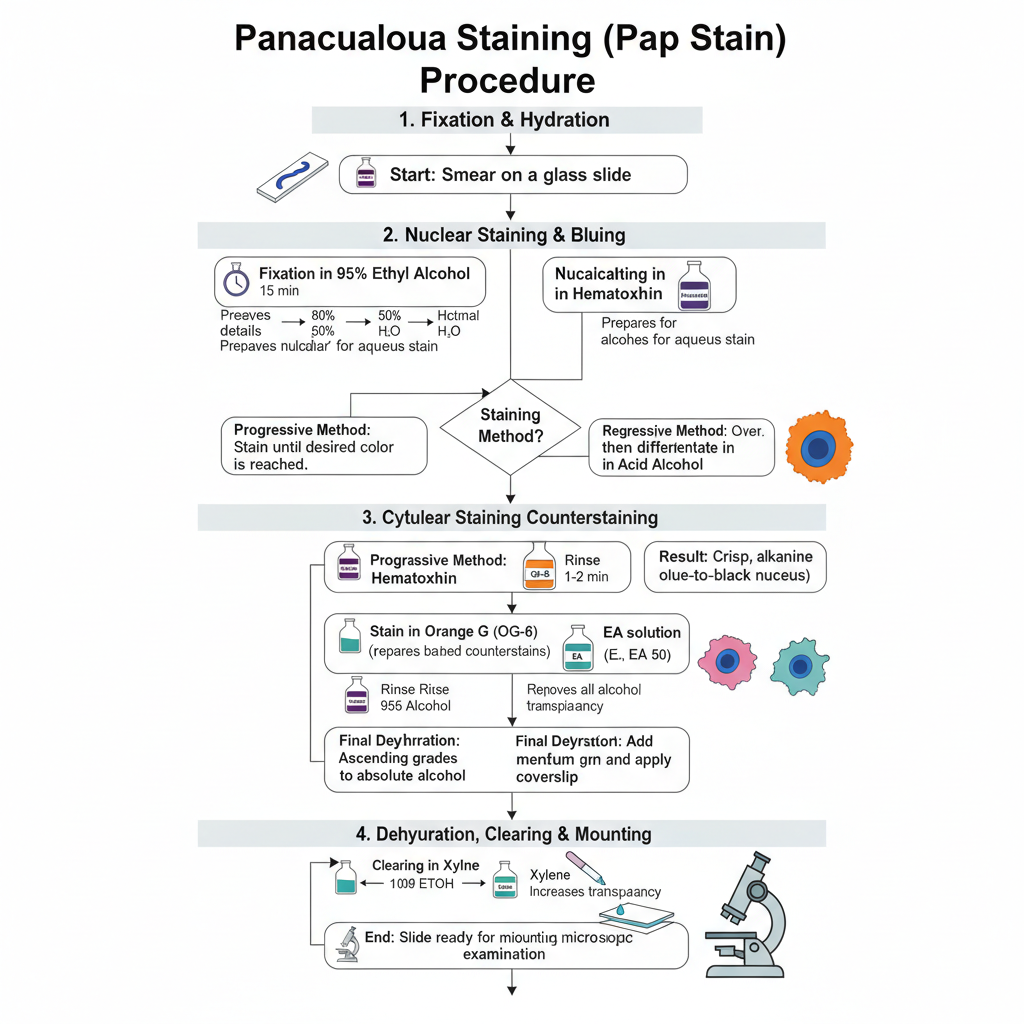
The Pap staining procedure is carried out in a definite sequence so that the nucleus and cytoplasm are stained separately with clear transparency. It is the process that begins with fixation, followed by nuclear staining, cytoplasmic staining and finally clearing and mounting. The steps are given below.
1. Fixation and Hydration
The smear is immediately fixed in 95% ethyl alcohol. It is kept for about 15 minutes so that the nuclear details are preserved because air drying causes swelling and distortion. After fixation, the slide is taken through descending grades of alcohol such as 95%, 80% and 50% and then brought to water. This hydration is required because hematoxylin is an aqueous stain.
2. Nuclear Staining and Bluing
The hydrated slide is put in hematoxylin stain for nuclear staining. Depending on the method used, the stain may be progressive where the slide is kept till the required colour is reached or regressive where the slide is overstained and then differentiated in dilute acid alcohol to remove excess stain. After staining, the slide is washed in an alkaline solution like Scott’s tap water or lithium carbonate. This is referred to as bluing and gives the nucleus a crisp blue to black appearance.
3. Cytoplasmic Counterstaining
Before cytoplasmic staining, the slide is again dehydrated to 95% alcohol because Orange G and EA dyes are alcohol based.
- The slide is stained in Orange G (OG-6) for 1–2 minutes. This stains keratinized cells in bright orange shades.
- The slide is rinsed in 95% alcohol to remove extra stain.
- The slide is then stained in EA solution (EA 36, EA 50 or EA 65) for about 2.5–10 minutes. The EA mixture contains Eosin Y and Light Green, and the cytoplasm appears pink or blue-green depending on the cell maturity.
4. Dehydration, Clearing and Mounting
After staining, the slide is passed through ascending grades of alcohol till absolute alcohol to remove water completely. Then the slide is placed in a clearing agent like xylene. It increases transparency of the specimen. Finally, a mounting medium is added and a coverslip is placed so that the slide is ready for microscopic examination.
Results and Interpretation of Papanicolaou Staining (Pap stain)
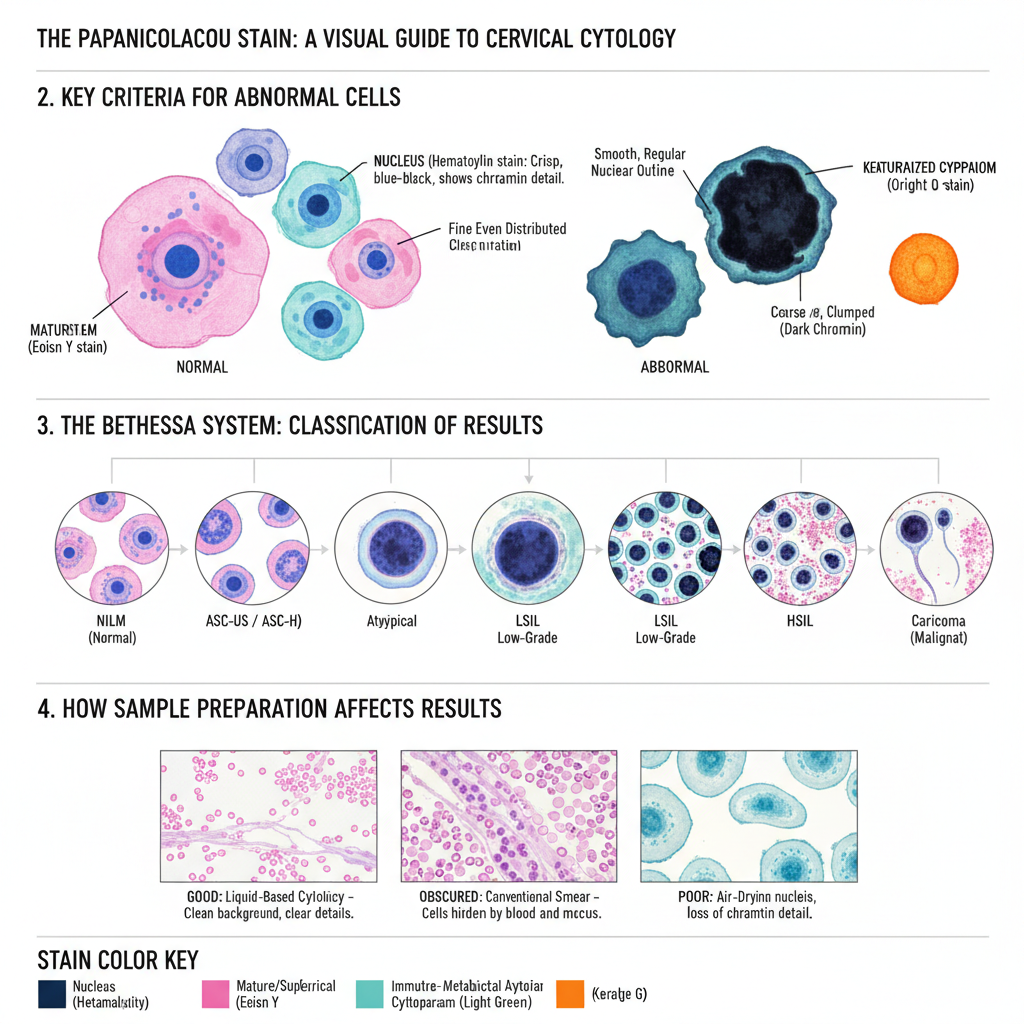
The stained smear shows a characteristic multicoloured appearance because each dye binds differently to the nuclear and cytoplasmic structures. It is the process in which the proper staining gives clear nuclear detail and transparent cytoplasm so that overlapping cells can also be examined. The nucleus appears blue to black, and the cytoplasm takes different shades like pink, green or orange depending on the maturity and metabolic activity of the cells.
Nuclear Staining
The nucleus is stained by hematoxylin and appears crisp blue to black. This colour is important because the chromatin pattern, nuclear margin and any irregularity is clearly seen. The nuclear clarity is the main feature that helps in identifying precancerous or cancerous changes.
Cytoplasmic Staining
Orange G and EA solutions stain the cytoplasm according to the type of cell.
- Metabolically active cells like parabasal and intermediate squamous cells and glandular cells appear blue-green due to Light Green dye.
- Mature superficial cells take pink shades because of Eosin Y.
- Keratinized cells appear bright orange because Orange G stains keratin strongly.
Criteria for Abnormal Cells
Interpretation mainly depends on the nucleus, as nuclear changes show early signs of malignancy. Some of the important features are–
- Nuclear hyperchromasia, where the nucleus becomes very dark.
- Irregular nuclear outline showing notches or variation in shape.
- Increase in nuclear–cytoplasmic ratio, where the size of the nucleus becomes larger compared to the cytoplasm.
- Coarse or uneven chromatin distribution.
- Presence of abnormal mitosis which sometimes indicate malignant transformation.
Classification of Results (Bethesda System)
The reporting follows the Bethesda System.
- NILM: Cells appear normal with regular nucleus and cytoplasm.
- ASC-US / ASC-H: Cells show slight atypia. ASC-US shows unclear changes, while ASC-H indicates changes suspicious for high-grade lesion.
- LSIL: Mild dysplasia where cells may show koilocytosis and nuclear enlargement.
- HSIL: Moderate to severe dysplasia. These cells are small, less mature and have high N/C ratio with hyperchromatic nucleus.
- Carcinoma: Malignant cells appear in irregular forms like spindle or tadpole shape, and background may show necrotic debris.
Effect of Sample Preparation
The appearance of the smear is influenced by how the sample is prepared. Conventional smears may contain blood, mucus or inflammatory cells that sometimes hide cellular details. Liquid-based cytology gives a cleaner background and produces a uniform layer of cells. Air-drying before fixation causes swelling of nuclei and loss of chromatin pattern, leading to poor staining and unsatisfactory results.
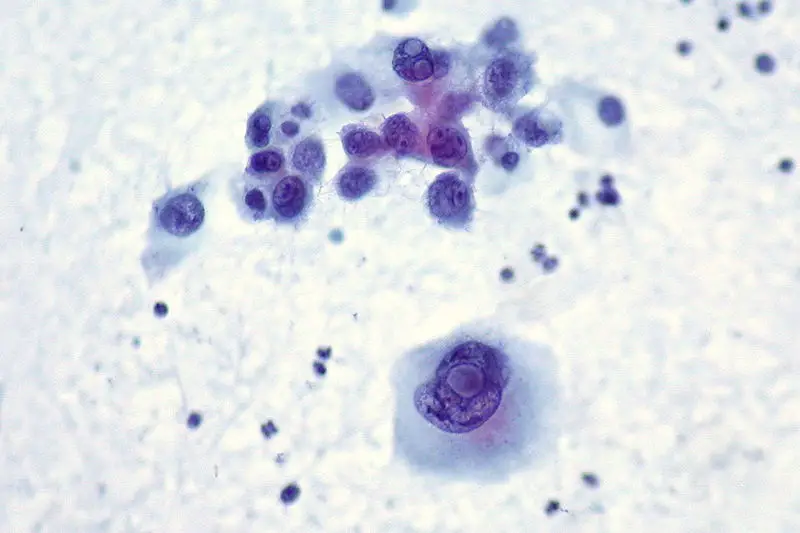
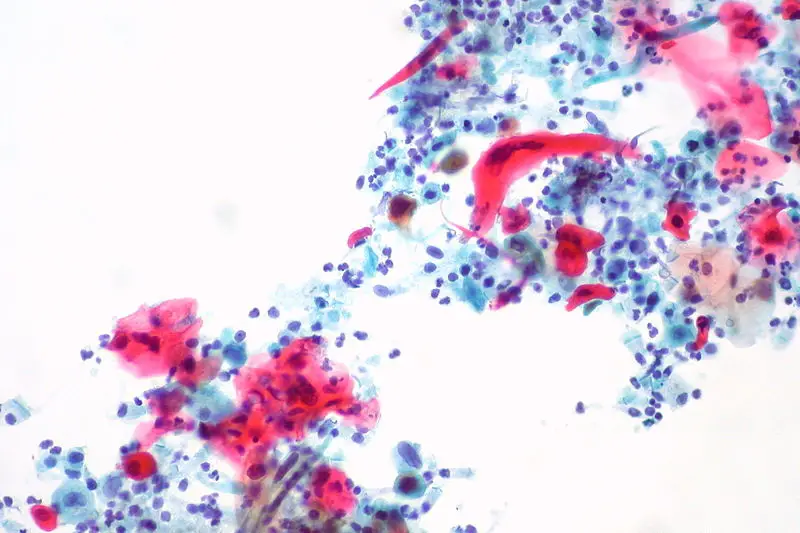
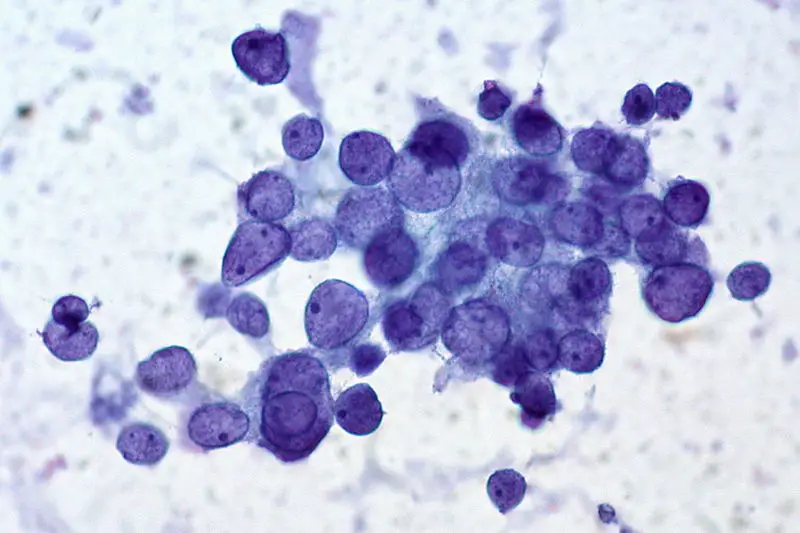
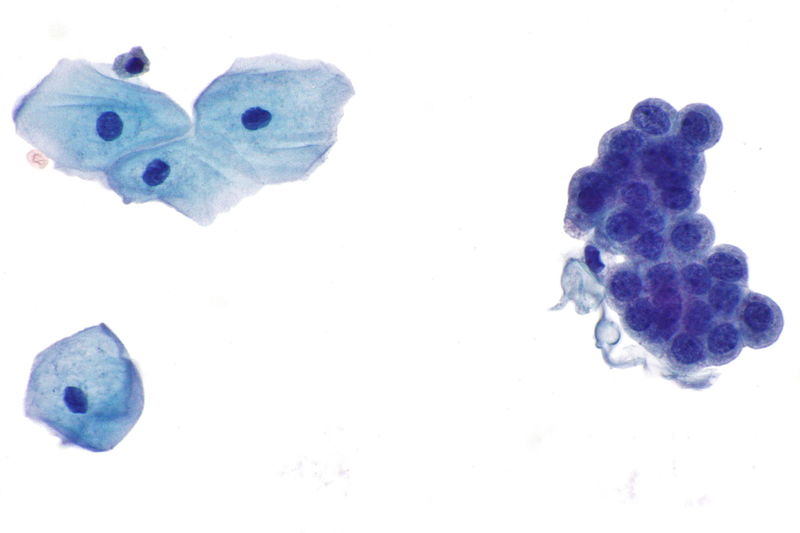
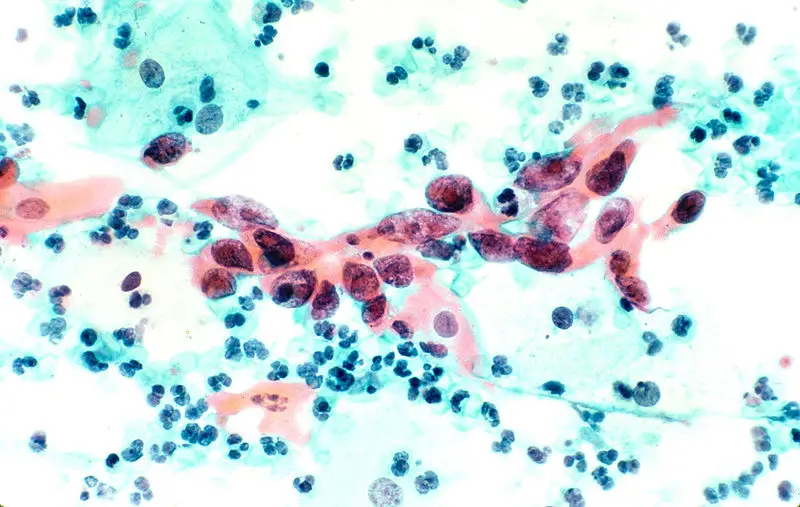
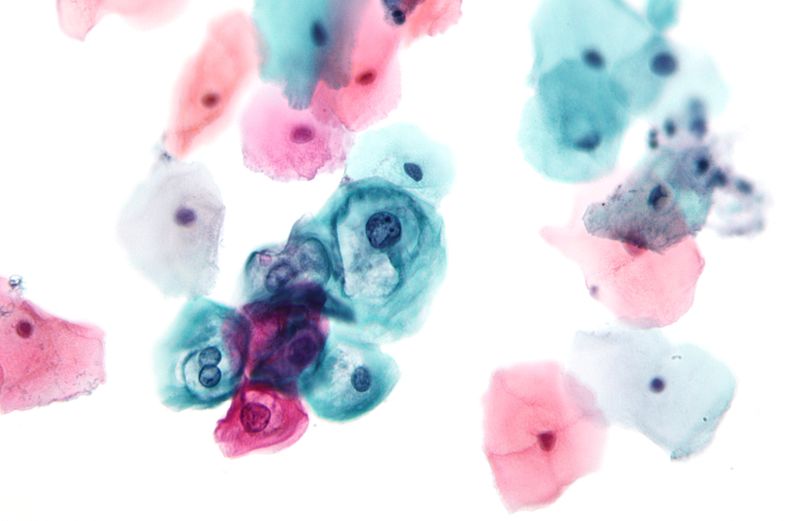
Uses of Papanicolaou Staining (Pap stain)
- It is used in cervical cancer screening to detect premalignant and malignant changes in squamous cells.
- It helps in identifying infections such as HPV-related koilocytes, Trichomonas vaginalis, Candida species and other organisms.
- It is used for hormonal evaluation, where the maturity of cells indicates estrogen or progesterone influence.
- It is applied in respiratory cytology for sputum, bronchial washings and BAL samples to detect lung cancers and respiratory infections.
- It is used in fine needle aspiration cytology of organs like thyroid, breast, lymph nodes and pancreas for studying nuclear details in tumour cells.
- It is used in examining pleural, peritoneal and other body cavity fluids to detect metastatic cells or mesothelioma.
- It is used in urinary cytology for diagnosing urothelial carcinoma from voided urine or bladder washings.
- It is applied for studying cerebrospinal fluid samples to detect metastatic spread or inflammatory conditions.
- It is also used for semen evaluation and other non-gynecological cytology where clear nuclear and cytoplasmic details are required.
Limitations of Papanicolaou Staining (Pap stain)
- It requires immediate wet fixation, and any delay causes air-drying artifacts which distort the nucleus and affect interpretation.
- The staining procedure is long and complex, and differences in dye composition like EA-36, EA-50 and EA-65 can cause variation in results.
- Some dyes such as Light Green or Bismarck Brown may become unstable or form precipitates, reducing the quality of staining solutions.
- Conventional smears may have blood, mucus or inflammatory cells that hide epithelial cells and make the smear difficult to evaluate.
- Uneven cell distribution in conventional smears sometimes leads to thick areas with overlapping cells.
- Sampling errors occur when some of the collected material is lost during transfer to the slide.
- False-negative results may appear because of inadequate sampling or screening errors, and abnormalities may be missed.
- Interpretation depends on the cytopathologist’s judgment, and high workload may lead to screening errors.
- Results such as ASC-US produce ambiguity because the changes do not fulfil criteria for dysplasia.
- The process uses a large quantity of alcohol and laboratory resources which may not be easily available in all settings.
- Conventional smears cannot be used for additional molecular tests, and a separate sample may be required for further evaluation.
Advantages of Papanicolaou Staining (Pap stain)
- It produces clear cytoplasmic transparency, allowing the nucleus to be observed even when the cells are overlapping.
- The multicoloured staining helps in differentiating metabolically active cells which appear blue-green and mature cells which take pink or orange shades.
- It gives sharp blue to black nuclear staining that helps in checking chromatin pattern, nuclear margin and other features important for diagnosing malignancy.
- It is useful not only in cervical smears but also in body fluids like urine, CSF, pleural and peritoneal fluids.
- It is widely used in respiratory cytology for sputum and bronchial samples where it helps in identifying malignant cells and keratinization.
- It is suitable for fine needle aspiration samples because the stain allows the evaluation of nuclear details in three-dimensional clusters.
- It is inexpensive and can be performed quickly, and the procedure does not damage the tissue so repeated sampling is possible.
- It helps in detecting infections such as Candida, Trichomonas, and viral changes along with assessing hormonal status depending on cell maturity.
FAQ
What is Papanicolaou staining (Pap stain)?
Papanicolaou staining (Pap stain) is a histological staining technique used to evaluate cellular material from cervical or vaginal smears for the diagnosis of cancer or other abnormal conditions.
What type of sample can be stained using Papanicolaou staining?
Papanicolaou staining is used on cervical or vaginal smears to detect abnormal cells.
How does Papanicolaou staining work?
Papanicolaou staining works by using dyes that bind to different components of cells, such as nuclei, cytoplasm, and mucus, to create a color-coded image of the sample.
Is Papanicolaou staining a qualitative or quantitative technique?
Papanicolaou staining is primarily a qualitative technique, as it is used to identify the morphological features of cells and diagnose abnormal conditions.
What is the sensitivity of Papanicolaou staining?
The sensitivity of Papanicolaou staining can vary, but it is generally considered to be high for detecting abnormal cells in cervical or vaginal smears.
What are the advantages of Papanicolaou staining?
The advantages of Papanicolaou staining include ease of use, low cost, high sensitivity for detecting abnormal cells, and widespread availability.
What are the disadvantages of Papanicolaou staining?
The disadvantages of Papanicolaou staining include potential for false negatives or false positives, subjectivity in interpretation, and the need for trained professionals to interpret the results.
Is Papanicolaou staining the only test used to detect abnormal cells in cervical or vaginal smears?
No, Papanicolaou staining is often used in conjunction with other diagnostic tests, such as HPV testing, to detect abnormal cells in cervical or vaginal smears.
What is the cost of Papanicolaou staining?
The cost of Papanicolaou staining can vary depending on the location and type of test, but it is generally considered to be low cost compared to other diagnostic tests.
How long does it take to get the results of a Papanicolaou stain?
The time it takes to get the results of a Papanicolaou stain can vary, but it is usually within a few days to a week after the sample is collected.
- Andola, S. K., Andola, U. S., Andola, S. S., Antony, A. T., Masgal, M., Patil, A. G., & Andola, K. S. (2024). Should liquid-based cytology (LBC) be preferred than conventional Pap smear (CPS): A comparative analysis. The Journal of Obstetrics and Gynecology of India, 74(4), 311–318. https://doi.org/10.1007/s13224-023-01828-x
- Canadian Cancer Society. (n.d.). Abnormal cervical biopsy results. https://cancer.ca/en/cancer-information/cancer-types/cervical/diagnosis/abnormal-cervical-biopsy-results
- Carl Zeiss Microscopy GmbH. (2018). A quick guide to cytological staining [Technology Note]. https://asset-downloads.zeiss.com/catalogs/download/mic/227d4c3d-457e-46f0-8e6a-3347aa84dbc5/EN_wp_Cytological-Staining.pdf
- Cleveland Clinic. (2024, August 19). Pap smear. https://my.clevelandclinic.org/health/diagnostics/4267-pap-smear
- ERemedium. (n.d.). Pap stain procedure. https://eremedium.in/pap-stain-procedure/
- Ethos Biosciences. (n.d.). How to set-up and conduct a Papanicolaou stain for both GYN and non-GYN samples. https://www.ethosbiosciences.com/how-to-set-up-and-conduct-a-papanicolaou-stain
- Goel, G., Halder, A., Joshi, D., Anil, A. C., & Kapoor, N. (2020). Rapid, economic, acetic acid Papanicolaou stain (REAP): An economical, rapid, and appropriate substitute to conventional Pap stain for staining cervical smears. Journal of Cytology, 37(4), 170–173. https://doi.org/10.4103/JOC.JOC_89_20
- Gurina, T. S., & Simms, L. (2023). Histology, staining. In StatPearls. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK557663/
- HealthSky Biotechnology Co., Ltd. (2025, May 13). How to interpret Pap stain results correctly. https://www.healthskybio.com/how-to-interpret-pap-stain-results-correctly.html
- IHC World. (2024, January 26). Papanicolaou stain (Pap stain) protocol. https://ihcworld.com/2024/01/26/papanicolaou-stain-pap-stain-protocol/
- Kamal, M. (2022). Pap smear collection and preparation: Key points. CytoJournal, 19, 24. https://doi.org/10.25259/CMAS_03_05_2021
- Kapse, S. S., Arakeri, S. U., & Yerranguntla, D. P. (2018). Rehydration of air-dried smears with normal saline: An alternative for conventional wet fixation method in cervical cytological study. Journal of Cytology, 35(4), 199–203. https://doi.org/10.4103/JOC.JOC_186_17
- Khieu, M., & Butler, S. L. (2023). High-grade squamous intraepithelial lesion of the cervix. In StatPearls. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK430728/
- Lata, Kumari, M., Kolte, S., & Arora, R. (2020). Removal of air-drying artifact of Papanicolaou stained smears with normal saline and fresh frozen plasma. Annals of Pathology and Laboratory Medicine, 7(3), A142–146. https://doi.org/10.21276/apalm.2724
- Makde, M. M., & Sathawane, P. (2022). Liquid-based cytology: Technical aspects. CytoJournal, 19, 41. https://doi.org/10.25259/CMAS_03_16_2021
- Malhotra, S., Kazlouskaya, V., Andres, C., Gui, J., & Elston, D. (2013). Diagnostic cellular abnormalities in neoplastic and non-neoplastic lesions of the epidermis: A morphological and statistical study. Journal of Cutaneous Pathology, 40(4). https://doi.org/10.1111/cup.12090
- Marshall, P. N. (1983). Papanicolaou staining–a review. Microscopica Acta, 87(3), 233–243.
- Mayo Clinic Staff. (2024, July 20). Pap smear. Mayo Clinic. https://www.mayoclinic.org/tests-procedures/pap-smear/about/pac-20394841
- Miller & Zois, Attorneys at Law. (n.d.). Pap smear misdiagnosis lawsuits. https://www.millerandzois.com/medical-malpractice/cancer-misdiagnosis-lawyer-verdicts/pap-smear-misdiagnosis/
- Naib, Z. M. (1990). Pap test. In H. K. Walker, W. D. Hall, & J. W. Hurst (Eds.), Clinical methods: The history, physical, and laboratory examinations (3rd ed.). Butterworths. https://www.ncbi.nlm.nih.gov/books/NBK287/
- National Cancer Institute. (n.d.-a). Cervical cancer screening. National Institutes of Health. https://www.cancer.gov/types/cervical/screening
- National Cancer Institute. (n.d.-b). HPV and Pap test results: Next steps after an abnormal cervical cancer screening test. National Institutes of Health. https://www.cancer.gov/types/cervical/screening/abnormal-hpv-pap-test-results
- WebPath. (n.d.). Histotechniques: Tissue processing. University of Utah. https://webpath.med.utah.edu/HISTHTML/HISTOTCH/HISTOTCH.html
- Weill Cornell Medicine Samuel J. Wood Library. (2011, June 29). George Papanicolaou: Development of the Pap smear. https://library.weill.cornell.edu/archives-blog/george-papanicolaou-development-pap-smear
- Wikipedia. (n.d.-a). Georgios Papanikolaou. Retrieved from https://en.wikipedia.org/wiki/Georgios_Papanikolaou
- Wikipedia. (n.d.-b). Papanicolaou stain. Retrieved from https://en.wikipedia.org/wiki/Papanicolaou_stain
- Wisconsin State Laboratory of Hygiene. (n.d.). Abnormal lesions. University of Wisconsin-Madison. https://www.slh.wisc.edu/clinical/cytology/resources-for-health-care-professionals/gynecologic-cytology-101/abnormal-precancerous-lesions-of-the-female-reproductive-system/