Ouchterlony Double Immunodiffusion Method is a qualitative immunological technique that is used to study antigen–antibody reaction in vitro. It is the process in which both antigen and antibody are allowed to diffuse freely in a semi-solid medium usually agar or agarose gel. This method was developed by Örjan Ouchterlony and it is commonly used for detection of antigenic relationship and comparison of different antigens. It is carried out on a glass slide or petri plate containing agar gel in which small wells are cut.
It is called double immunodiffusion because both the antigen and antibody diffuses towards each other through the gel medium. In this process antigen solution is placed in one well and antibody (antiserum) is placed in the adjacent well. As diffusion continues, the antigen and antibody meet at a point where their concentration is optimal. At this zone of equivalence, antigen–antibody complex is formed which is insoluble and appears as a visible white precipitin line in the gel. The formation of this line indicates the presence of specific antigen–antibody reaction.
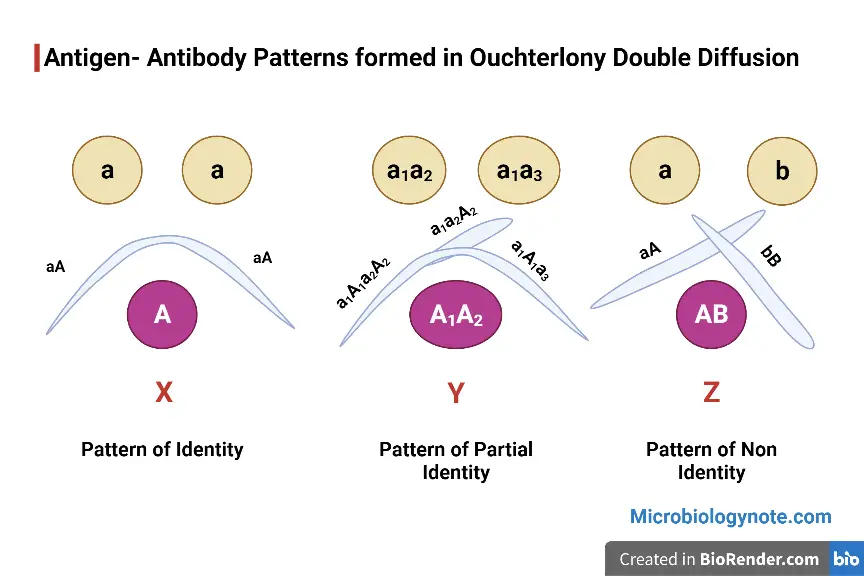
By observing the pattern of precipitin lines, the immunological relationship between antigens can be determined. If the precipitin lines fuse smoothly it indicates identity, crossed lines indicate non-identity and formation of spur indicates partial identity. This method is simple and does not require sophisticated instruments. Although more advanced techniques are available, Ouchterlony double immunodiffusion method is still used for confirmatory tests and for understanding basic antigen–antibody interactions.
Objectives of Ouchterlony Double Immunodiffusion Method
- To detect the presence of antigen–antibody reaction by formation of visible precipitin lines in agar gel.
- To compare different antigens and determine their antigenic relationship such as identity partial identity and non-identity.
- To identify specific antigen or antibody present in a given biological or clinical sample.
- To study the relative concentration of antigen or antibody based on position of precipitin line formed in the gel.
- To help in diagnosis of certain autoimmune and infectious diseases by confirming specific antibodies.
- To determine toxigenicity of bacterial strains by observing toxin–antitoxin reaction in gel.
- To demonstrate basic principles of immunology such as diffusion precipitation and lattice formation for educational purpose.
Principle of Ouchterlony Double Immunodiffusion
The Ouchterlony double immunodiffusion method is based on the principle of passive diffusion of antigen and antibody through a semi-solid medium such as agar or agarose gel. In this method wells are cut in the gel and antigen solution is placed in one well while antibody solution is placed in the adjacent well. Both antigen and antibody diffuses radially in all directions through the gel medium. When the diffusing antigen and antibody molecules meet each other at a suitable concentration ratio a specific antigen–antibody reaction takes place.
At the point where optimum concentration of antigen and antibody is present a zone of equivalence is formed. In this zone an insoluble antigen–antibody lattice is produced which appears as a visible white precipitin line in the gel. The position and pattern of this precipitin line depends on the nature and relationship of the antigens involved. Thus the formation of precipitin line confirms the specificity of antigen–antibody reaction and helps in qualitative analysis of immunological relationship.
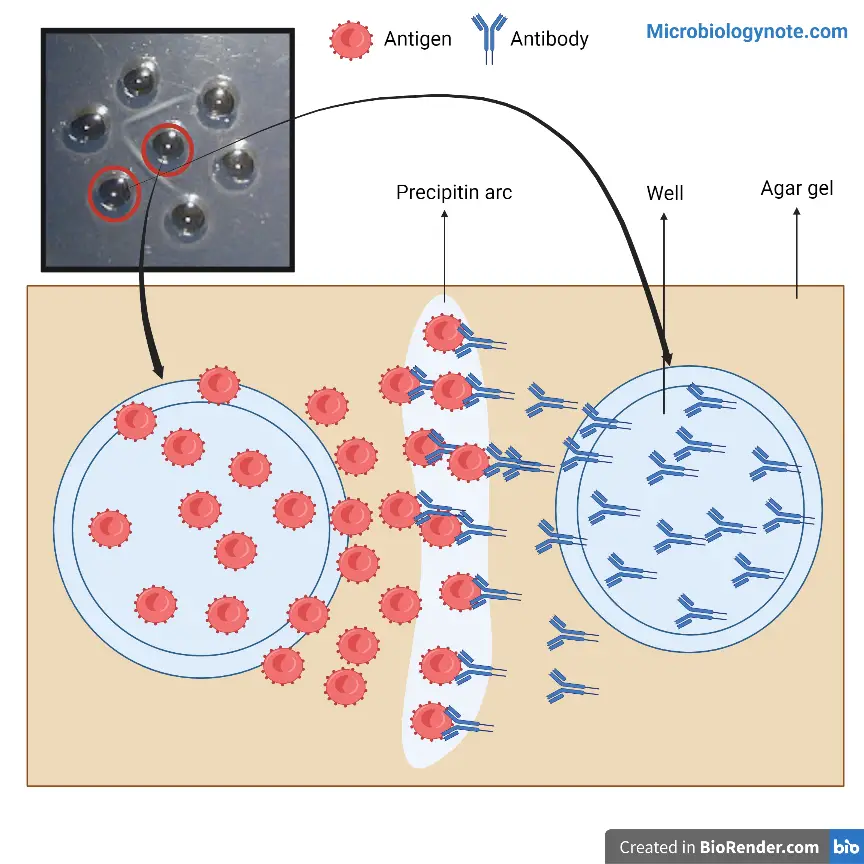
Materials Required
- Agar or agarose gel for preparation of semi-solid medium.
- Buffer solution such as phosphate buffer saline (PBS) for dissolving agar and maintaining pH.
- Antigen solutions to be tested.
- Antisera (antibody solution) specific to the antigen.
- Distilled water for preparation of reagents and washing purpose.
- Glass slides or petri dishes for pouring agar gel.
- Gel puncher or cork borer for making wells in agar gel.
- Micropipette with tips for loading antigen and antibody into wells.
- Template for proper arrangement and equal spacing of wells.
- Moist chamber or humid chamber to prevent drying of gel during diffusion.
- Heating source such as water bath or hot plate for melting agar.
- Staining and destaining solutions if precipitin lines are to be enhanced for observation.
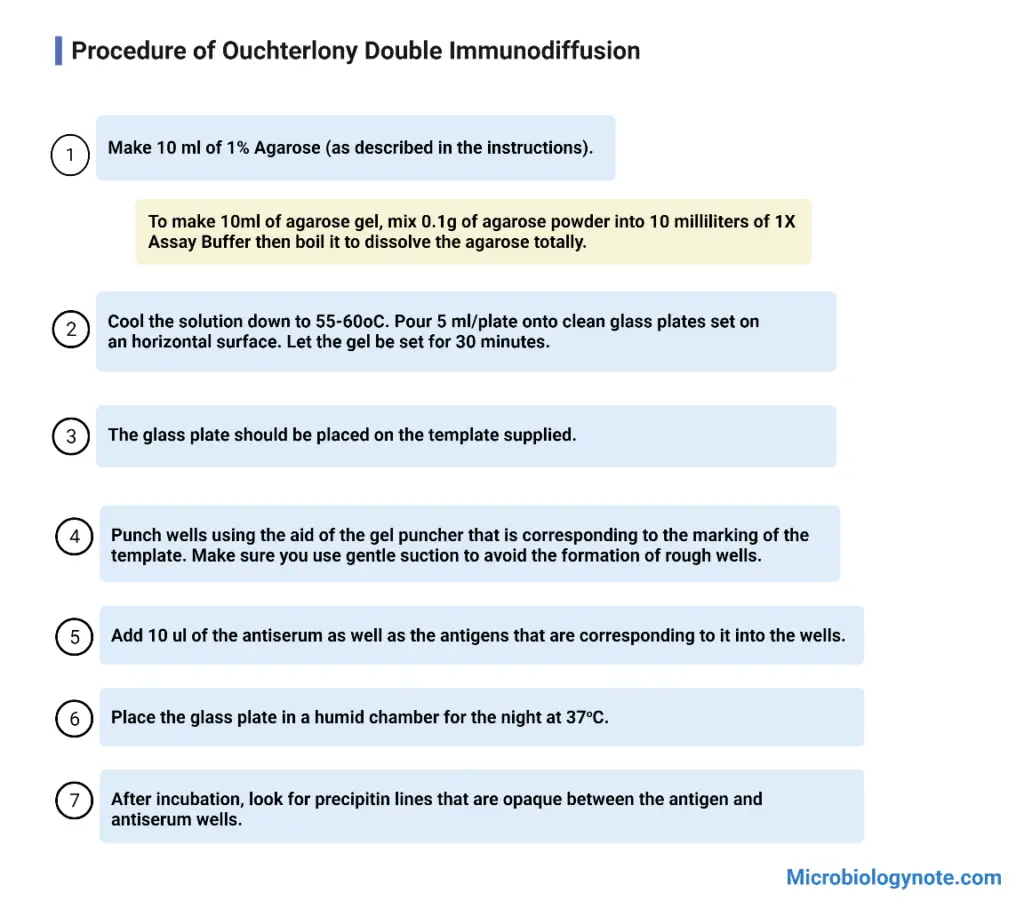
Procedure of Ouchterlony Double Immunodiffusion
- Agar or agarose is dissolved in buffer solution by heating until a clear solution is obtained.
- The molten agar is cooled slightly and poured on clean glass slide or petri plate to form a thin uniform gel layer. It is allowed to solidify at room temperature.
- After solidification wells are cut in the gel using a cork borer or gel puncher following a proper pattern. Agar plugs are removed carefully.
- Antigen solution is added into one well and antibody (antiserum) solution is added into the adjacent wells using a micropipette.
- The plate is placed in a moist chamber to prevent drying of gel and kept undisturbed at room temperature or 37°C for 24 to 48 hours.
- During incubation antigen and antibody diffuses through the gel and react with each other forming precipitin lines.
- After incubation the gel is observed for formation of visible white precipitin lines between the wells.
- If required the gel is washed dried and stained to make the precipitin lines more clearly visible.
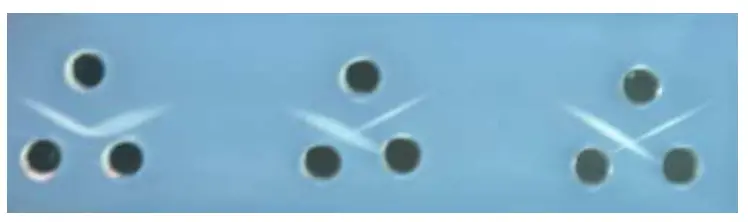
Results of Ouchterlony Double Immunodiffusion
- Formation of visible white precipitin line between antigen and antibody wells indicates positive antigen–antibody reaction.
- Absence of precipitin line indicates negative result and shows no reaction between antigen and antibody.
- Identity reaction is observed when precipitin lines from two antigen wells fuse smoothly forming a continuous arc which indicates immunologically identical antigens.
- Non-identity reaction is observed when precipitin lines cross each other forming an X-shaped pattern which indicates unrelated antigens.
- Partial identity reaction is observed when precipitin lines fuse but a spur is formed which indicates partially related antigens with some common determinants.
- Position of precipitin line between the wells gives an idea about relative concentration of antigen or antibody.
- Sharp and distinct precipitin line indicates strong and specific reaction while broad or multiple lines indicate weak reaction or presence of more than one antigen–antibody system.
Precautions of Ouchterlony Double Immunodiffusion Method
- Agar or agarose gel should be poured on a clean grease-free glass slide or petri plate kept on a perfectly level surface.
- Agar solution should be completely dissolved by proper heating and should be cooled slightly before pouring to avoid uneven gel formation.
- Wells should be cut carefully with smooth edges and equal distance should be maintained between the wells.
- Agar plugs must be removed gently without disturbing the surrounding gel.
- Antigen and antibody solutions should be added carefully into the wells without overflow or spillage on the gel surface.
- Separate micropipette tips should be used for each sample to avoid cross contamination.
- Air bubbles should be avoided while loading the samples into the wells.
- The gel plate should be kept in a moist chamber during incubation to prevent drying and cracking of the gel.
- The plate should not be disturbed during diffusion as movement may distort precipitin line formation.
- All antigen and serum samples should be handled carefully as they may be potentially infectious.
Zonal reaction
- Zone of equivalence is the region where antigen and antibody diffuses and meet at optimum concentration resulting in formation of visible precipitin line.
- Prozone reaction occurs when antibody is present in excess as compared to antigen. In this condition no precipitin line is formed due to lack of lattice formation.
- Postzone reaction occurs when antigen is present in excess over antibody. Here also no visible precipitate is formed because proper cross linking does not take place.
- Position of precipitin line depends upon relative concentration of antigen and antibody.
- When antigen concentration is high the precipitin line is formed nearer to antibody well.
- When antigen concentration is low the precipitin line is formed nearer to antigen well.
Applications of Ouchterlony Double Immunodiffusion
- It is used for diagnosis of fungal infections such as aspergillosis histoplasmosis blastomycosis and coccidioidomycosis.
- It is used in diagnosis of autoimmune diseases by detection of antibodies against extractable nuclear antigens (ENA).
- It is used to determine toxigenicity of bacterial strains by Elek test for Corynebacterium diphtheriae.
- It is used to study antigenic relationship between different antigens and to identify identity partial identity and non-identity reactions.
- It is used for semi-quantitative estimation of antigen or antibody concentration based on position of precipitin line.
- It is used to check purity and specificity of antigen and antibody preparations.
- It is used in forensic serology to identify species origin of blood and body fluid samples.
- It is used in taxonomy and evolutionary studies for comparison of protein antigens among different organisms.
- It is used in teaching laboratories to demonstrate antigen–antibody reaction and principles of immunodiffusion.
Advantages of Ouchterlony Double Immunodiffusion Method
- It is a simple and economical method which does not require costly instruments or electrical equipment.
- The technique is easy to perform and interpret and hence suitable for routine laboratory use.
- It helps in comparing different antigens and determining their antigenic relationship such as identity partial identity and non-identity.
- It is highly specific and gives reliable confirmatory results for antigen–antibody reaction.
- Antigen and antibody react in their native form without denaturation in the gel medium.
- Multiple antigen–antibody reactions can be studied simultaneously on a single gel plate.
- It provides a semi-quantitative idea about relative concentration of antigen or antibody.
- Results are directly visible as precipitin lines and can be preserved by staining for future reference.
Limitations of Ouchterlony Double Immunodiffusion Method
- It is a less sensitive method and requires high concentration of antigen and antibody for visible precipitin line formation.
- The procedure is time consuming and usually takes 24 to 48 hours for complete diffusion and result formation.
- It is mainly a qualitative method and provides only approximate estimation of antigen or antibody concentration.
- Interpretation of results depends on visual observation and may vary from person to person.
- Prozone and postzone effect may occur which can give false negative results.
- The method is not suitable for testing large number of samples at a time.
- Larger quantity of antigen and antibody is required as compared to modern immunological techniques.
- Abcam. (n.d.). Western blotting vs. ELISA: Choosing the right technique for accurate results. Retrieved from https://www.abcam.com
- Amrita Vishwa Vidyapeetham. (2011). Ouchterlony double diffusion – Patterns (Procedure). Immunology Virtual Lab II. vlab.amrita.edu
- Amrita Vishwa Vidyapeetham. (2011). Ouchterlony double diffusion – Patterns (Theory). Immunology Virtual Lab II. vlab.amrita.edu
- Amrita Vishwa Vidyapeetham. (2012). Ouchterlony double diffusion – Titration. Immunology Virtual Lab II. vlab.amrita.edu
- Azure Biosystems. (2023, February 17). When to use ELISA vs Western blot. https://azurebiosystems.com
- BenjaminMutisyaMuimi. (n.d.). DOUBLE IMMUNODIFFUSION METHO(mancini method)D.pptx. SlideShare.
- Carrillo, A. (2023, February 17). When to use ELISA vs Western blot. Azure Biosystems.
- Centurion University of Technology and Management. (n.d.). 6. Ouchterlony double immunodiffusion test. CUTM Courseware. https://courseware.cutm.ac.in
- Clinical Lab Products. (2001, July 1). Fungal immunodiffusion.
- CliniSciences. (n.d.). Immunodiffusion (ID) Assay for mycology.
- Dhemaji College. (n.d.). Immunodiffusion technique [PDF].
- Filo. (2025, October 13). Distinguish between mancini immunodiffusion and outchelony’s immunodiffusion.
- Fiveable Content Team. (2025, September). Immunodiffusion.
- Freidank, H., Thiel, L., & Henninger, S. (1994). Comparison of immunodiffusion and counterimmunoelectrophoresis for the detection of precipitating antibodies against Candida and Aspergillus antigens. Mycoses, 37(Suppl 1), 79–83.
- G-Biosciences. (n.d.). Antigen-antibody interactions: Teacher’s guidebook (Cat. # BE-501).
- Heidelberger, M., & Kendall, F. E. (1934). Quantitative studies on the precipitin reaction: The rôle of multiple reactive groups in antigen-antibody union as illustrated by an instance of cross-precipitation. Journal of Experimental Medicine, 59(4), 519–528. https://doi.org/10.1084/jem.59.4.519
- HiMedia Laboratories. (2014, May). HiPer® Ouchterlony Double Diffusion Teaching Kit (Antigen-Antibody Pattern) [Product Information].
- Høiby, N. (2024). Örjan Ouchterlony and the antigen–antibody double diffusion‐in‐gel: A survey. APMIS, 133(1), e13480. https://doi.org/10.1111/apm.13480
- Hornbeck, P. (2017). Double-immunodiffusion assay for detecting specific antibodies (Ouchterlony). Current Protocols in Immunology, 116(1), 2.3.1–2.3.4. https://doi.org/10.1002/cpim.18
- Key Diagnostics. (n.d.). FSK1 Aspergillus immunodiffusion system [Instructions for Use]. Microgen Bioproducts.
- MetwareBio. (n.d.). ELISA vs. Western blot: Choosing the best immunoassay for your research.
- Mujtaba, M. G., Baliban, T., Bhagu, J., & Herrera, M. (2021). A laboratory exercise simulating antibody and antigen reactions of the Ouchterlony double immunodiffusion assay using inorganic salts. Journal of Microbiology & Biology Education, 22(2), e00103-21. https://doi.org/10.1128/jmbe.00103-21
- Neupsy Key. (2016, June 4). Principles of analytical methods.
- Orton, S. M., Peace-Brewer, A., Schmitz, J. L., Freeman, K., Miller, W. C., & Folds, J. D. (2004). Practical evaluation of methods for detection and specificity of autoantibodies to extractable nuclear antigens. Clinical and Diagnostic Laboratory Immunology, 11(2), 297–301. https://doi.org/10.1128/CDLI.11.2.297-301.2004
- Pal, A., & Sen, S. (2025). Developing tools for learning immunology using diffusion‐based salt precipitation assays: A low‐cost alternative for college laboratories. Biochemistry and Molecular Biology Education, 53(4), 336–343. https://doi.org/10.1002/bmb.21900
- Precision Antibody. (2025, August 21). ELISA, Flow, Western: Choosing the right assay for your antibody.
- Suganya, S., & Alagumalai, S. K. (2025). Diagnostic accuracy of ELISA versus indirect immunofluorescence in detecting anti-nuclear antibodies among suspected connective tissue disorder patients. Journal of Contemporary Clinical Practice, 11(7), 490–495. https://doi.org/10.61336/jccp/25-07-65
- Taylor & Francis. (n.d.). Ouchterlony double immunodiffusion. Taylor & Francis Knowledge.
- Thermo Fisher Scientific. (2024, January 11). ELISA vs Western blot: When to use each immunoassay technique. Life in the Lab.
- Wikipedia contributors. (2024). Fick’s laws of diffusion. Wikipedia, The Free Encyclopedia.
- Wikipedia contributors. (2024). Ouchterlony double immunodiffusion. Wikipedia, The Free Encyclopedia.
- Wikipedia contributors. (2024). Örjan Ouchterlony. Wikipedia, The Free Encyclopedia.
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.