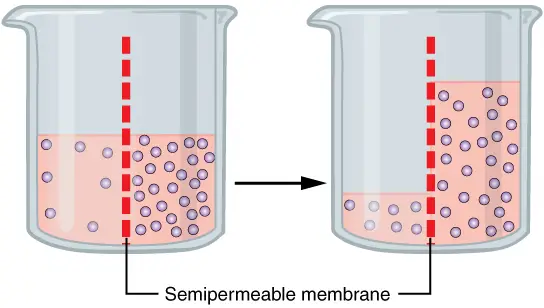
What is Osmosis?
- Osmosis is a fundamental biological process defined as the spontaneous movement of solvent molecules through a selectively permeable membrane. This movement occurs from a region of high water potential, characterized by lower solute concentration, to a region of low water potential, where solute concentration is higher. The ultimate goal of osmosis is to equalize solute concentrations on both sides of the membrane, facilitating equilibrium. This phenomenon is essential for maintaining homeostasis in biological systems.
- In more technical terms, osmosis refers specifically to the movement of water across membranes that permit the passage of water but restrict the movement of solutes. The concept of osmotic pressure is crucial here; it is the external pressure needed to halt the net movement of solvent molecules across the membrane. This pressure is a colligative property, which means it is determined by the solute’s molar concentration rather than its specific identity. Thus, various solutes can exert different osmotic pressures depending on their concentrations in a solution.
- Biological membranes are inherently semipermeable, meaning they allow certain molecules to pass while blocking others. Generally, these membranes prevent the passage of large and polar molecules, such as ions and proteins, while permitting non-polar or hydrophobic molecules, as well as small gases like oxygen and carbon dioxide, to pass. The permeability of a membrane is influenced by several factors, including solute size, charge, and solubility.
- Water plays a crucial role in osmosis, as it is the most common solvent in biological systems. Water molecules travel through the plasma membrane, tonoplast membrane, or organelle membranes by diffusing across the phospholipid bilayer. This process is often facilitated by specialized proteins called aquaporins, which function similarly to ion channels, allowing for efficient water transport.
- Osmosis serves several vital functions in biological contexts. For instance, it is the primary mechanism by which cells regulate water content. When a cell is placed in a hypotonic solution (one with lower solute concentration), water flows into the cell, leading to increased turgor pressure. Conversely, in a hypertonic solution (higher solute concentration), water exits the cell, resulting in a decrease in turgor pressure and potential cell shrinkage.
- A practical demonstration of osmosis can be observed with potato slices placed in saline solutions. When these potato slices are submerged in a concentrated salt solution, water moves out of the cells into the surrounding solution, causing the potato to lose turgor pressure and shrink. The degree of shrinkage correlates with the concentration of the salt solution: the higher the concentration, the greater the loss of water and subsequent reduction in size.
- The history of osmosis can be traced back to ancient observations, with significant advancements made over time. The first formal documentation of osmosis occurred in 1748 by Jean-Antoine Nollet. The term “osmosis” itself is derived from the Greek words for “push” and “within,” reflecting the movement of water molecules driven by concentration gradients. In 1867, Moritz Traube’s invention of selective precipitation membranes significantly improved the understanding and measurement of osmotic flow, further solidifying the importance of this phenomenon in both biological and chemical contexts.
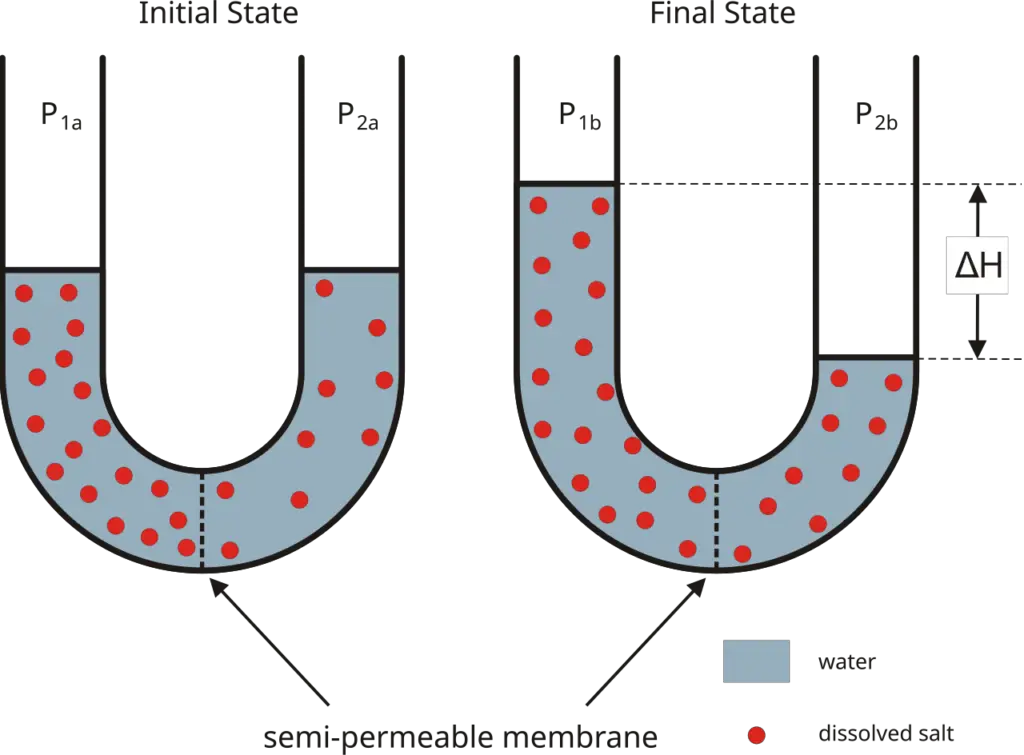
Definition of Osmosis
Osmosis is the spontaneous movement of solvent molecules, typically water, through a selectively permeable membrane from a region of lower solute concentration to a region of higher solute concentration, aiming to equalize solute concentrations on both sides.
How Does Osmosis Work?
Osmosis is a fundamental process that facilitates the movement of water across a selectively permeable membrane. It occurs when two solutions with differing solute concentrations are separated by this membrane. Understanding the mechanisms behind osmosis involves exploring the forces that drive water movement and the structural components involved in the process.
- Movement Across Membranes: In osmosis, water molecules move from a region of high water concentration (low solute concentration) to a region of low water concentration (high solute concentration). This movement continues until an equilibrium is reached, where there is no net flow of water.
- Role of Semipermeable Membranes: A critical requirement for osmosis is the presence of a semipermeable membrane. Such membranes allow water molecules to pass while restricting the movement of solute molecules. Without this membrane, the process resembles diffusion rather than osmosis.
- Concentration Gradient: The driving force behind osmosis is the concentration gradient of water across the membrane. Water moves downhill, meaning it seeks to equalize the concentration differences by flowing toward areas with higher solute concentrations.
- Chemical Potential: The concept of chemical potential is essential to understanding osmosis. The chemical potential of pure water differs from that of water in a solution containing solute molecules. The presence of solute reduces the pressure exerted by water molecules, creating a difference in potential.
- Pressure and Equilibrium: As water molecules move from the pure water side to the solute side, they exert pressure that continues until the chemical potential equilibrates. This pressure results from the water molecules in the pure solution pushing toward the area with a higher solute concentration.
- Osmotic Pressure Gradient: Osmosis is driven by the osmotic pressure gradient, which is the difference in osmotic pressures between the two solutions. This gradient dictates the tendency of water molecules to migrate from one area to another.
- Water Potential: The relative tendency of water to move is often quantified by water potential, denoted by the Greek letter Ψ (Psi). Water potential is a measure that encompasses both solute potential and pressure potential, influencing the direction and extent of water movement.
- Channel Proteins: Water molecules, being polar, require assistance to traverse cell membranes effectively. Channel proteins embedded within the membrane provide hydrophilic pathways, facilitating the movement of water along the concentration gradient. These proteins are vital for the efficient transport of water, ensuring that osmosis can occur swiftly and effectively.
Factors Affecting Osmosis
Osmosis, the movement of solvent molecules across a selectively permeable membrane, is influenced by several critical factors. Understanding these factors helps elucidate how osmosis operates in various biological and chemical contexts.
- Temperature: The rate of osmosis is directly influenced by temperature. As the temperature of a system increases, the kinetic energy of the molecules also rises. This heightened energy results in increased molecular movement, thereby accelerating the process of osmosis. Consequently, higher temperatures facilitate faster rates of water movement across the membrane.
- Concentration Gradient: The concentration gradient is a pivotal driving force in osmosis. When there is a significant difference in solute concentration across the membrane, osmosis is expedited. In a scenario where one solution contains a higher concentration of solute than another, the pressure exerted by the solvent molecules decreases in the more concentrated solution. This decline in pressure enhances the movement of solvent molecules toward the area of higher solute concentration. However, once equilibrium is achieved and solute concentrations equalize, the net movement of water ceases.
- Water Potential/Solvent Potential: The water potential across a semi-permeable membrane is another determinant of the osmosis rate. Solutions with higher water potential allow for increased movement of water molecules across the membrane, as the pressure exerted by the particles within the solution is greater. As the osmotic movement continues, water potential will eventually equilibrate on both sides of the membrane. Once equilibrium is established, water molecules continue to flow, but in equal amounts, maintaining a stable balance between the solutions.
- Surface Area and Membrane Thickness: The characteristics of the membrane also significantly impact the rate of osmosis. An increase in surface area enhances the available space for molecular movement, thus facilitating a higher rate of osmosis. Conversely, a reduction in surface area limits molecular movement and restricts the process. Furthermore, the thickness of the membrane plays a crucial role; as membrane thickness increases, the rate of osmosis tends to decrease due to the longer diffusion pathway for water molecules.
- Pressure: Pressure is a fundamental factor affecting osmosis. When external pressure is applied to the system, it can influence the direction of osmotic movement. If the pressure exceeds the natural pressure exerted by the solvent molecules, the direction of osmosis may reverse, causing solvent molecules to migrate toward the region with higher solvent concentration. Conversely, if the applied pressure is less than that exerted by the solvent molecules, it does not change the direction but can reduce the overall rate of osmosis. When pressure is applied in the same direction as the concentration gradient, it can enhance the rate of osmosis by facilitating increased movement of solvent molecules.
Types of Osmosis
Osmosis can be categorized into two distinct types based on the direction of solvent movement relative to the cell: endosmosis and exosmosis. Each type of osmosis plays a crucial role in maintaining cellular homeostasis and influencing cell behavior in different environments.
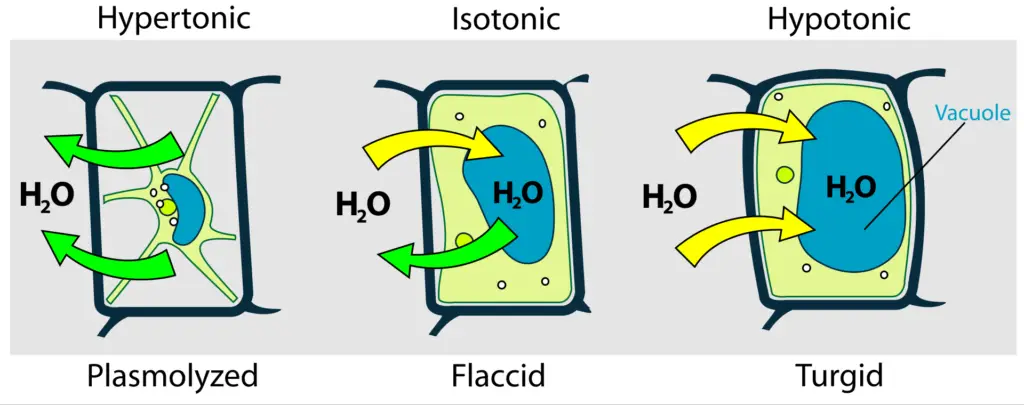
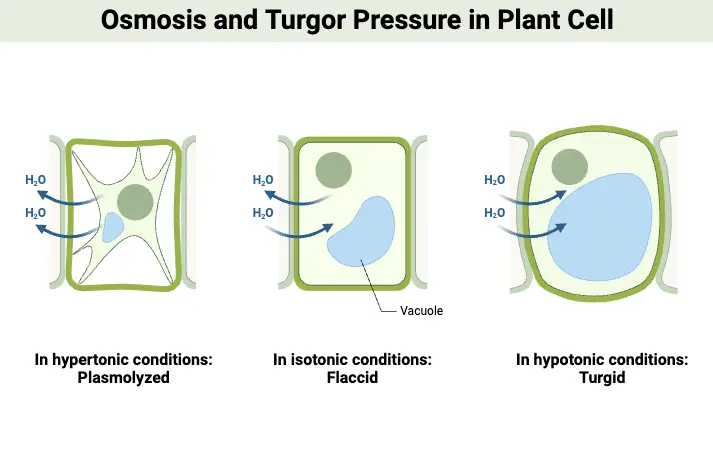
- Endosmosis: This type of osmosis occurs when a cell is placed in a hypotonic solution, which has a lower solute concentration compared to the cytoplasm of the cell. In this scenario, solvent molecules, typically water, move into the cell. As water enters, the cell absorbs this influx and swells, becoming turgid. This phenomenon is crucial for plant cells, as turgidity helps maintain structural integrity and support. The process of endosmosis can also be described as deplasmolysis, where the cell regains water after having previously lost it, thus restoring its normal shape and functionality.
- Exosmosis: Conversely, exosmosis takes place when a cell is immersed in a hypertonic solution, characterized by a higher solute concentration relative to the cell’s interior. In this situation, solvent molecules exit the cell, leading to a loss of water. As a result, the cell becomes flaccid, undergoing plasmolysis, which can impair its functions and lead to cell shrinkage. Exosmosis is significant in various physiological processes, such as the regulation of water balance in cells and tissues.
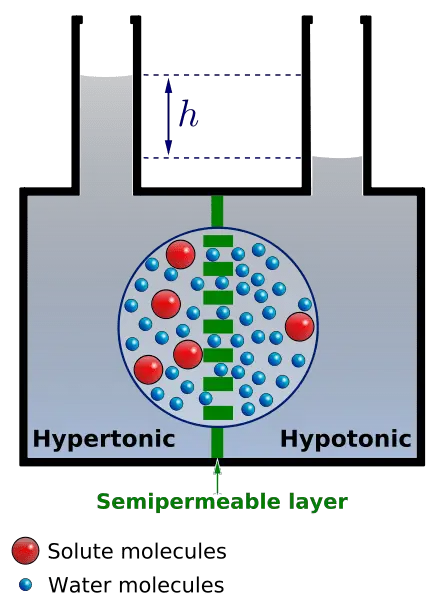
What is Osmotic Solution (Tonicity)?
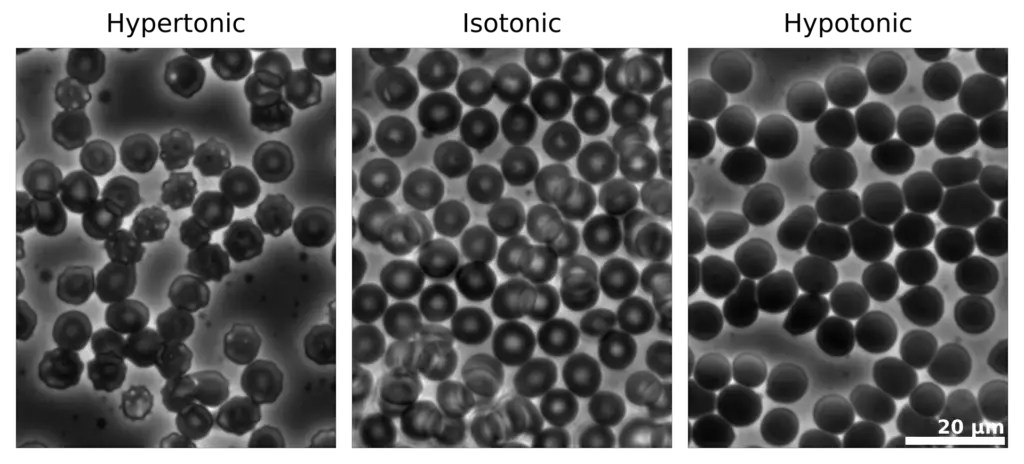
Tonicity refers to the ability of extracellular solutions to influence the movement of water across cell membranes through the process of osmosis. It is fundamentally determined by the concentration of solute and solvent molecules in a solution, which dictates how water will flow in relation to the cell’s internal environment. Solutions can be classified into three categories based on their tonicity: hypotonic, hypertonic, and isotonic.

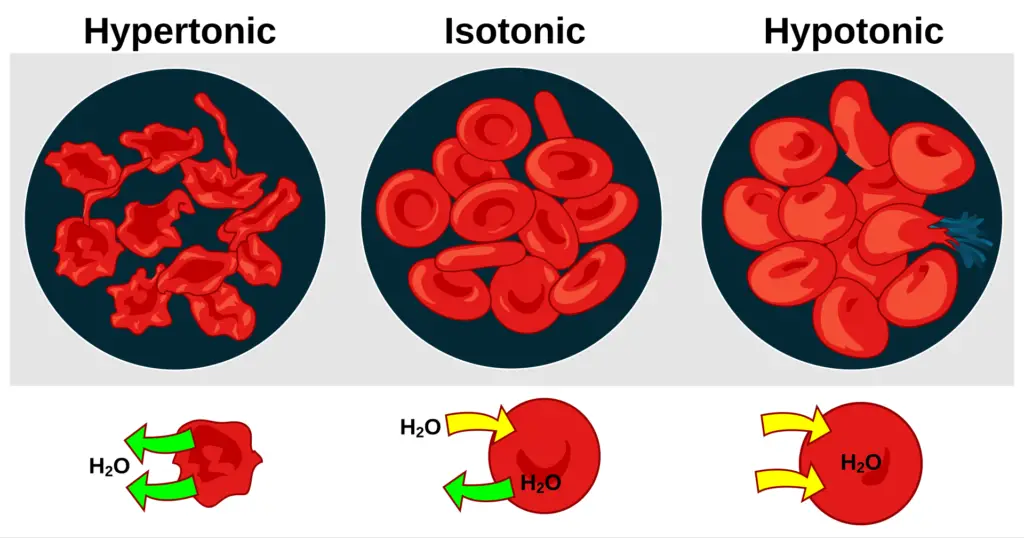
- Hypotonic Solution: A solution is considered hypotonic when it contains a lower concentration of solute compared to the interior of a cell. In this context, water moves into the cell, leading to endosmosis. As a result, the cell may swell and potentially burst due to the increased internal pressure. This phenomenon is crucial for plant cells, which rely on turgor pressure for structural integrity.
- Hypertonic Solution: Conversely, a hypertonic solution has a higher concentration of solute compared to the cell’s interior. When a cell is placed in a hypertonic solution, water exits the cell, causing exosmosis. This movement results in cell shrinkage, impairing its ability to function and divide. This process is significant in various physiological contexts, including cellular dehydration.
- Isotonic Solution: An isotonic solution has an equal concentration of solute as that found within the cell. In such scenarios, there is no net movement of water across the cell membrane, leading to a stable cell size. This balance is essential for maintaining homeostasis within the cell, as it ensures that cellular functions can proceed without disruption from osmotic stress.
Variation of Osmosis
Osmosis is a vital process that varies in its mechanisms and applications, particularly in its forward and reverse forms. These variations highlight the versatility of osmotic principles in different contexts, such as water purification and nutrient separation.
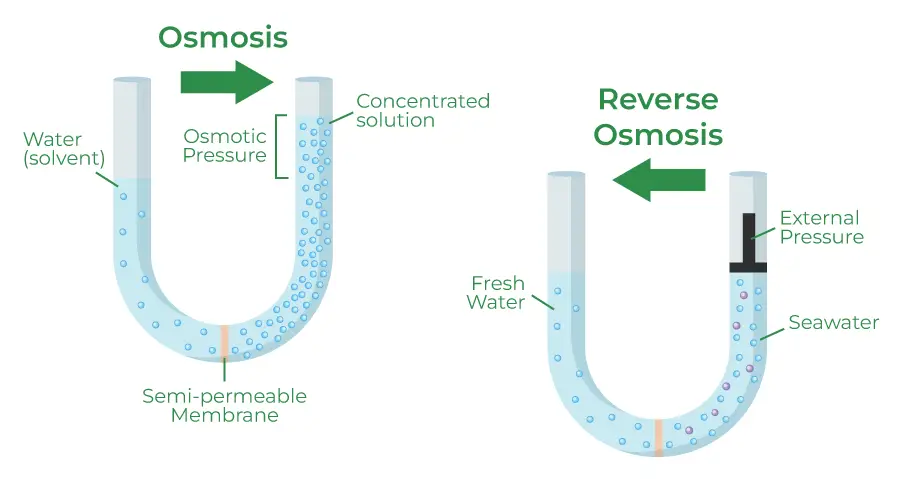
- Reverse Osmosis:
- Reverse osmosis involves applying pressure to drive a solvent through a semipermeable membrane, effectively filtering out solutes. In this process, pure solvent is forced from an area of higher solute concentration to an area of lower solute concentration.
- The pressure applied must exceed the osmotic pressure of the solution, allowing the selective separation of water while retaining dissolved substances on one side of the membrane. This method is widely utilized for water purification and desalination, enabling the production of potable water from saline or contaminated sources.
- Forward Osmosis:
- Forward osmosis operates on the principle of using a draw solution with higher osmotic pressure than the feed solution to separate water from a mixture containing undesirable solutes. In this variation, water moves through a semipermeable membrane towards the draw solution, resulting in the dilution of the draw solution and the concentration of the feed solution.
- The movement of water induced by the osmotic pressure gradient leads to a net flow that can be advantageous in various applications. The diluted draw solution can either be used directly or undergo a secondary separation process to extract the draw solute, which can often be more efficient compared to reverse osmosis alone.
What is Osmotic Pressure?
Osmotic pressure is a fundamental concept in biology and chemistry, representing the pressure required to prevent the flow of solvent molecules across a semi-permeable membrane. This pressure is crucial for regulating the movement of water in biological systems, influencing cell hydration and homeostasis. It serves as the driving force behind osmosis, where solvent molecules move from areas of lower solute concentration to areas of higher solute concentration.
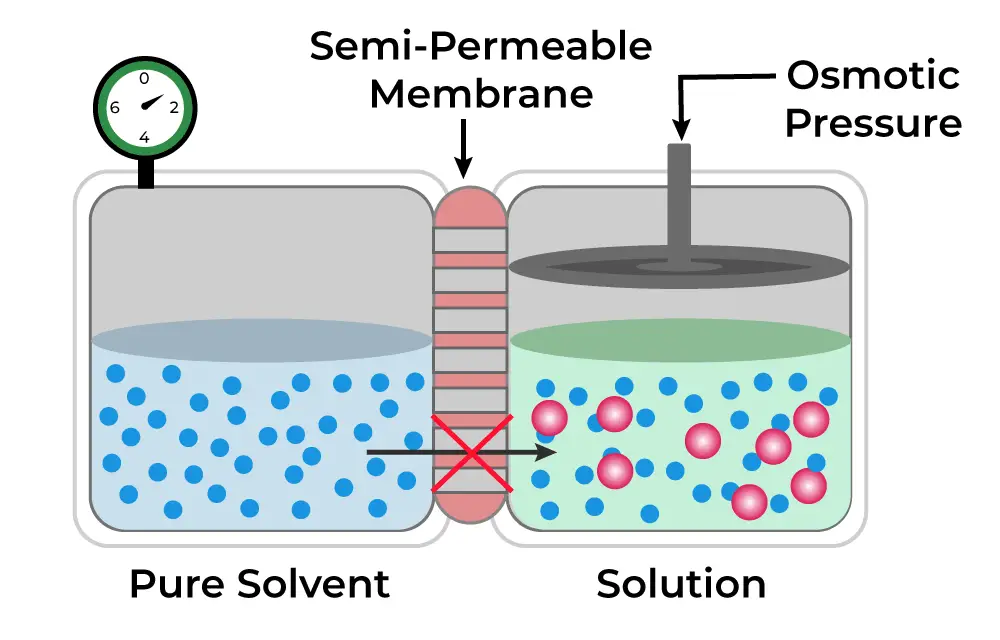
- Definition: Osmotic pressure is defined as the minimum pressure needed to halt the inward flow of pure solvent into a solution through a semi-permeable membrane. This membrane allows the passage of solvent molecules while restricting solute molecules, creating a concentration gradient that drives osmotic movement.
- Influence of Solute Concentration: The osmotic pressure of a solution is directly related to the concentration of solute particles present. As the concentration of solute increases, the osmotic pressure also rises, leading to a greater driving force for osmosis. Therefore, a more concentrated solution exerts a higher osmotic pressure, compelling solvent molecules to move toward it.
- Calculation: Osmotic pressure can be quantitatively expressed using the equation: ∏= MRT
In this formula:- ∏ represents the osmotic pressure,
- M denotes the molar concentration of solute particles,
- R is the gas constant, and
- T indicates the absolute temperature of the system (in Kelvin).
- Role in Biological Systems: Osmotic pressure is vital for maintaining cellular integrity and function. For example, it influences the movement of water in and out of cells, affecting cell turgidity in plants and the hydration state of animal cells. Cells must balance osmotic pressure to avoid excessive swelling or shrinking, which can compromise their viability.
Significance of Osmosis
Osmosis is a crucial process that significantly influences various biological and chemical systems. It facilitates the movement of water and nutrients within living organisms, maintaining cellular functions and overall health. The role of osmosis extends beyond simple water transport, impacting physiological processes critical to both plants and animals.
- Nutrient Transport: Osmosis is responsible for the absorption of essential nutrients and water from the soil into plant roots. This movement occurs through osmosis, allowing plants to transport water upward through the xylem to various parts of the plant, ensuring adequate hydration for metabolic activities.
- Cellular Homeostasis: The internal environment of living cells is stabilized by osmosis, which maintains a balance between water and intracellular fluid levels. This balance is vital for cellular functions, preventing conditions that could lead to cell damage or death.
- Turgidity Maintenance: Osmosis plays a key role in maintaining cell turgor, the pressure of the cell contents against the cell wall. Turgid cells support plant structures, enabling them to remain upright and facilitating growth. This turgidity is essential for the movement of plant parts, influencing how plants respond to environmental stimuli.
- Prevention of Desiccation: In plants, osmosis prevents the drying out of cells due to water loss during transpiration. By regulating internal water levels, plants can retain moisture, which is crucial for their survival, especially in arid environments. Additionally, osmosis helps prevent the desiccation of fruits and sporangia, ensuring successful reproduction and dispersal.
- Response to Environmental Stress: Osmosis enables plants in drought conditions to withstand water scarcity. Increased osmotic pressure within cells can help them maintain hydration, providing resilience against extreme environmental conditions.
- Water Movement and Regulation: Osmosis facilitates the diffusion of water and other cellular fluids between cells, regulating the internal movement of these substances. This process is critical for nutrient uptake, waste expulsion, and maintaining overall cellular health.
- Role in Seed Germination: During seed germination, osmosis is vital as it allows water to enter seeds, initiating metabolic processes necessary for growth. The uptake of water activates enzymes that facilitate the transformation of stored nutrients into usable forms for the developing plant.
- Physiological Mechanisms: Osmosis influences various physiological processes, including the functioning of stomata, where the movement of water molecules and the response of guard cells to osmotic pressure regulate gas exchange and transpiration rates.
- Applications in Water Purification: Beyond biological systems, osmosis has practical applications in methods such as reverse osmosis and forward osmosis. These techniques are utilized for the purification of drinking water, desalination, wastewater treatment, and even in the concentration of liquid foods.
Examples of Osmosis
Osmosis is a fundamental biological process that influences the movement of water across semipermeable membranes, affecting both animal and plant cells in various ways. Understanding these examples helps illustrate the critical role osmosis plays in maintaining cellular integrity and function.
- Osmosis in Animal Cells:
- When animal cells are exposed to a hypertonic solution—where the concentration of solute is higher outside the cell—water exits the cells. This leads to a phenomenon known as crenation, where the cells shrink due to water loss. For example, red blood cells (RBCs) will shrink in size when placed in diluted blood plasma.
- Conversely, when animal cells are placed in a hypotonic solution, characterized by a lower concentration of solute, water moves into the cells. This influx can cause the cells to swell, and if the osmosis continues unchecked, it may lead to the cells bursting.
- Another illustrative example is observed in slugs; when these animals come into contact with salt, which creates a hypertonic environment, the water is drawn out from their cells. This results in the slugs shrinking, demonstrating the effects of osmotic pressure on their semi-permeable skin.
- Osmosis in Plant Cells:
- Plant cells exhibit a different response to osmotic changes due to the presence of rigid cell walls. When placed in a hypotonic solution, plant cells take up water, leading to increased turgor pressure, which helps maintain the plant’s upright structure. This pressure is essential for supporting the plant and facilitating growth.
- However, when plant cells are exposed to a hypertonic solution, water moves out of the cells. Although the cell wall provides some structural support, the loss of water causes the cells to shrink and become flaccid. This can impact the plant’s ability to function optimally.
- A practical example of osmosis in plants is seen in the root system, where root cells absorb water from the soil through their semi-permeable membranes. This process is crucial for delivering water to various parts of the plant, influencing physiological functions.
- Additionally, the behavior of potato cells serves as a classic illustration of osmosis. When potato slices are immersed in a hypotonic solution, the cells swell as they absorb water, while placing them in a hypertonic solution results in the cells losing water and shrinking.
Difference Between Osmosis and Diffusion
Osmosis and diffusion are fundamental processes that describe the movement of molecules, yet they differ significantly in their mechanisms and specific applications. Understanding these differences is crucial for comprehending various biological and chemical phenomena.
- Definition:
- Osmosis is a specialized form of diffusion that specifically involves the movement of solvent molecules, predominantly water, through a semipermeable membrane. This movement occurs from an area of lower solute concentration to an area of higher solute concentration.
- In contrast, diffusion refers to the general process of particles moving from a region of higher concentration to one of lower concentration. This process continues until equilibrium is established, where the concentration of particles is uniform throughout the medium.
- Particles Involved:
- Osmosis typically involves only solvent molecules, such as water, which traverse the semipermeable membrane.
- Diffusion can involve a variety of particles, including solute molecules and gases, and can occur in various media, such as liquids, gases, or solids.
- Selective Barrier:
- Osmosis requires a semipermeable membrane that selectively allows certain molecules to pass while restricting others. This membrane plays a crucial role in determining the direction and extent of water movement.
- Diffusion can occur in the absence of any membrane. In an open system, particles can move freely without any barrier.
- Direction of Movement:
- In osmosis, the movement of solvent molecules is directed towards areas of higher solute concentration, contributing to the dilution of that area until equilibrium is reached.
- Diffusion involves the movement of particles from regions of higher concentration to those of lower concentration until an even distribution is achieved.
- Energy Requirement:
- Both osmosis and diffusion are generally passive processes that do not require energy input from the system. Osmosis is categorized as passive transport because it relies on the concentration gradient.
- While diffusion is predominantly passive, certain forms, such as facilitated diffusion, may involve specific protein channels and can require minimal energy to assist in the movement of particles.
Study of Osmosis by Potato Osmometer
The potato osmometers serve as effective models for studying osmosis, illustrating the principles of endosmosis and exosmosis using simple materials. This experiment provides clear insights into the movement of water through semipermeable membranes, represented by the potato tuber.
- Materials Required:
- Peeled potato
- Knife
- Pure water
- Concentrated sugar solution
- Petri plate
- Pins
- Procedure:
- Preparation of the Potato: Start by peeling a large potato using a knife. This allows for the creation of a suitable structure to observe osmotic activity.
- Shaping the Potato: Cut the upper and lower portions of the peeled potato to ensure stability when placed on the Petri plate. This provides a flat base for the potato.
- Creating a Cavity: Use a knife to carve out a cavity in the center of the potato, extending it deep enough to hold the sugar solution but leaving a small thickness at the bottom. This bottom section acts as a selective membrane.
- Setting Up the Experiment: Position the potato on the Petri plate.
- Endosmosis Experiment: To study endosmosis, pour pure water into half of the Petri plate. Simultaneously, pour concentrated sugar solution into half of the potato cavity.
- Exosmosis Experiment: For exosmosis, place concentrated sugar solution in the Petri plate and add water to the cavity of the potato tuber.
- Marking Levels: Insert pins into the potato tuber at points A and B to indicate the initial levels of sugar solution and water in the cavity, respectively.
- Observation Period: Allow the setup to remain undisturbed for a designated time to observe any changes in the levels of the liquids.
- Observation:
- For Endosmosis: In the cavity of potato tuber A, observe that the level of the sugar solution increases. This indicates that water from the Petri plate is moving into the potato tuber, where there is a higher solute concentration, leading to the conclusion that water moves towards areas of lower solvent concentration.
- For Exosmosis: In the cavity of potato tuber B, note that the level of water decreases. This occurs because the water moves out of the potato tuber into the concentrated sugar solution on the Petri plate, demonstrating that water exits the cell to balance solute concentrations in the surrounding environment.
- Results:
- The increase in sugar solution in tuber A confirms endosmosis, while the decrease in water in tuber B supports the concept of exosmosis, showcasing the directional movement of water based on solute concentrations.
- Precautions:
- Ensure that the cavity is deep enough to hold the solutions while leaving an adequate thickness at the bottom of the potato to maintain structural integrity.
- Utilize a concentrated sugar solution to create a significant osmotic gradient necessary for clear observation of osmotic processes.
- Lopez MJ, Hall CA. Physiology, Osmosis. [Updated 2023 Mar 13]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557609/
- https://www.biologyonline.com/dictionary/osmosis
- https://www.geeksforgeeks.org/osmosis/
- https://biologyreader.com/study-of-osmosis-by-potato-osmometer.html
- https://www.futurelearn.com/info/courses/teaching-biology-inspiring-students-with-plants-in-science/0/steps/58750
- https://www.priyamstudycentre.com/2022/09/osmosis.html
- https://senecalearning.com/en-GB/definitions/osmosis/
- https://en.wikipedia.org/wiki/Osmosis
- https://byjus.com/biology/osmosis/#types