Nylander’s test is a qualitative test that is used for detecting reducing sugars. It is the process where the reducing sugar is heated with an alkaline solution containing bismuth salt, and the reaction is controlled by the components present in the reagent. The reagent is made of bismuth nitrate or bismuth subnitrate, potassium sodium tartrate, and a strong alkali (NaOH or KOH). It is the tartrate which helps in preventing the formation of insoluble bismuth hydroxide in the alkaline medium. In this condition the reducing sugar form an enediol and this enediol acts on the bismuth(III) ion. The bismuth(III) is reduced to elemental bismuth which appear as a black or dark brown precipitate after heating. This is referred to as the positive reaction of the test.
Nylander’s test was used for detecting glucose in urine samples, and it is the process which was considered very sensitive but the reaction is also affected by other reducing compounds like uric acid or creatinine. Because of this the test is not used commonly and the modern enzymatic methods is now used in clinical practice.
Purpose of Nylander’s Test
- It is the test used for detecting reducing sugars in a solution by observing formation of black metallic bismuth after heating.
- The test helps to identify sugars like glucose, fructose and other reducing carbohydrates because these sugars reduce the bismuth salt in alkaline medium.
- It was previously used for checking glucose in urine, so it served as an early method for detecting glucosuria in suspected diabetes.
- It also demonstrate the reducing property of carbohydrates under strong alkali and heat, showing how sugar convert into reactive form which reduce metal ions.
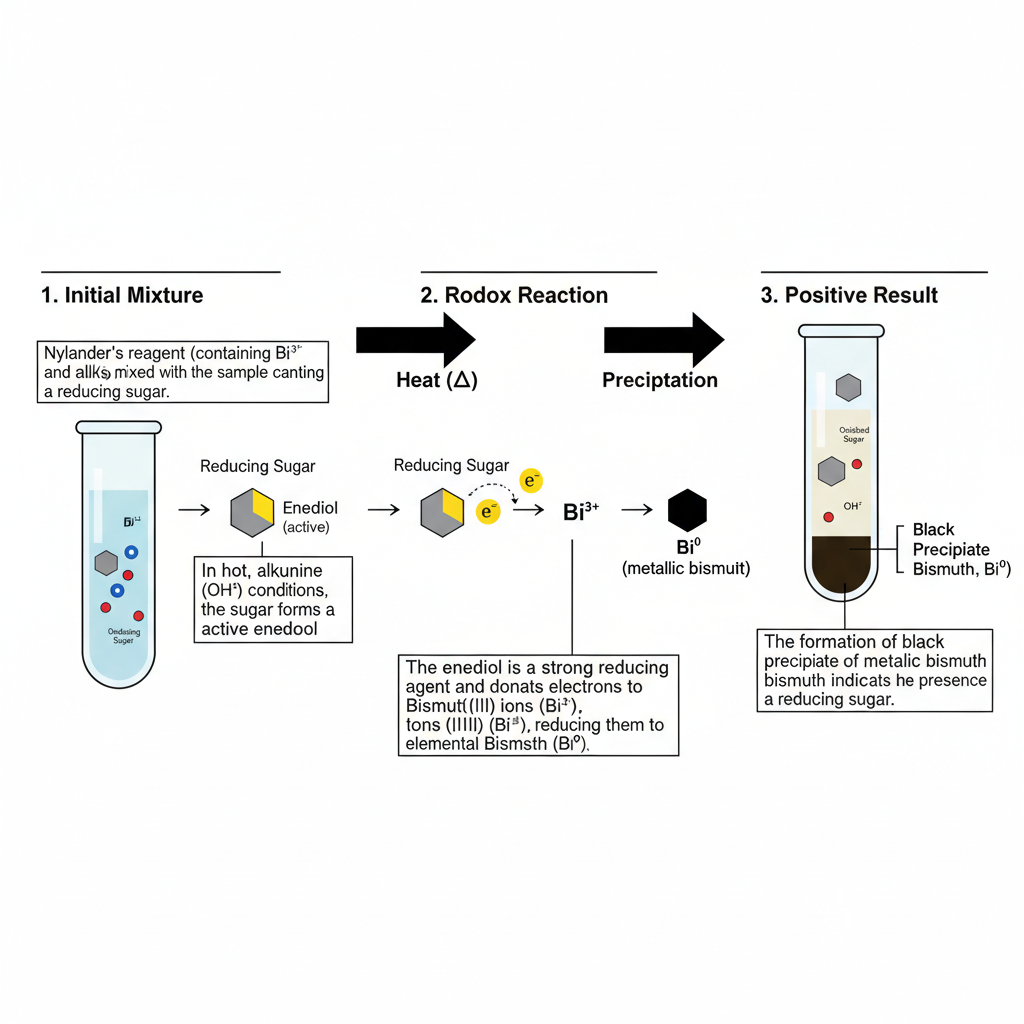
Principle of Nylander’s Test
The principle of Nylander’s test is based on the property of reducing sugars which can reduce bismuth(III) ions into elemental bismuth when the reaction is carried out in strong alkaline condition. It is the process where the reducing sugar having a free aldehyde or ketone group is converted into an enediol form in the presence of alkali. This enediol is the active compound which donate electrons to the bismuth salt. The reagent contains bismuth subnitrate or bismuth oxynitrate as the oxidizing agent, potassium sodium tartrate which keep the bismuth ion in solution, and a strong alkali like NaOH or KOH that create the high pH needed for the reaction. In this condition the tartrate prevents the formation of insoluble bismuth hydroxide, so the Bi³⁺ ion remain available for reduction. When the mixture is heated, the bismuth(III) is reduced to metallic bismuth (Bi⁰) and the black or dark brown precipitate is formed. This is referred to as the positive reaction of the test although other reducing compounds can also produce similar reaction.
Requirements for Nylander’s Test
- A sample solution which is suspected to contain reducing sugar is required, mainly glucose or fructose.
- Nylander’s reagent is needed and it is freshly prepared before using.
- The reagent contain three major components –
- Bismuth nitrate / Bismuth subnitrate – it is reduced by the sugar to form metallic bismuth.
- Potassium sodium tartrate (Rochelle salt) – it help to keep bismuth ions in solution by forming tartrate complex and also stop formation of insoluble bismuth hydroxide.
- Potassium hydroxide (KOH) or Sodium hydroxide (NaOH) – it gives the alkaline condition needed for activation of sugar molecule.
- Test tube, test tube stand and pipette is required for handling and mixing.
- A heat source like water bath or spirit lamp is essential since the reaction is occurred only by heating.
- The mixture is usually boiled for around 2–5 minutes until colour change or black precipitate is formed.
- All glassware must be clean and dry before using, otherwise the reaction is not showing proper result.
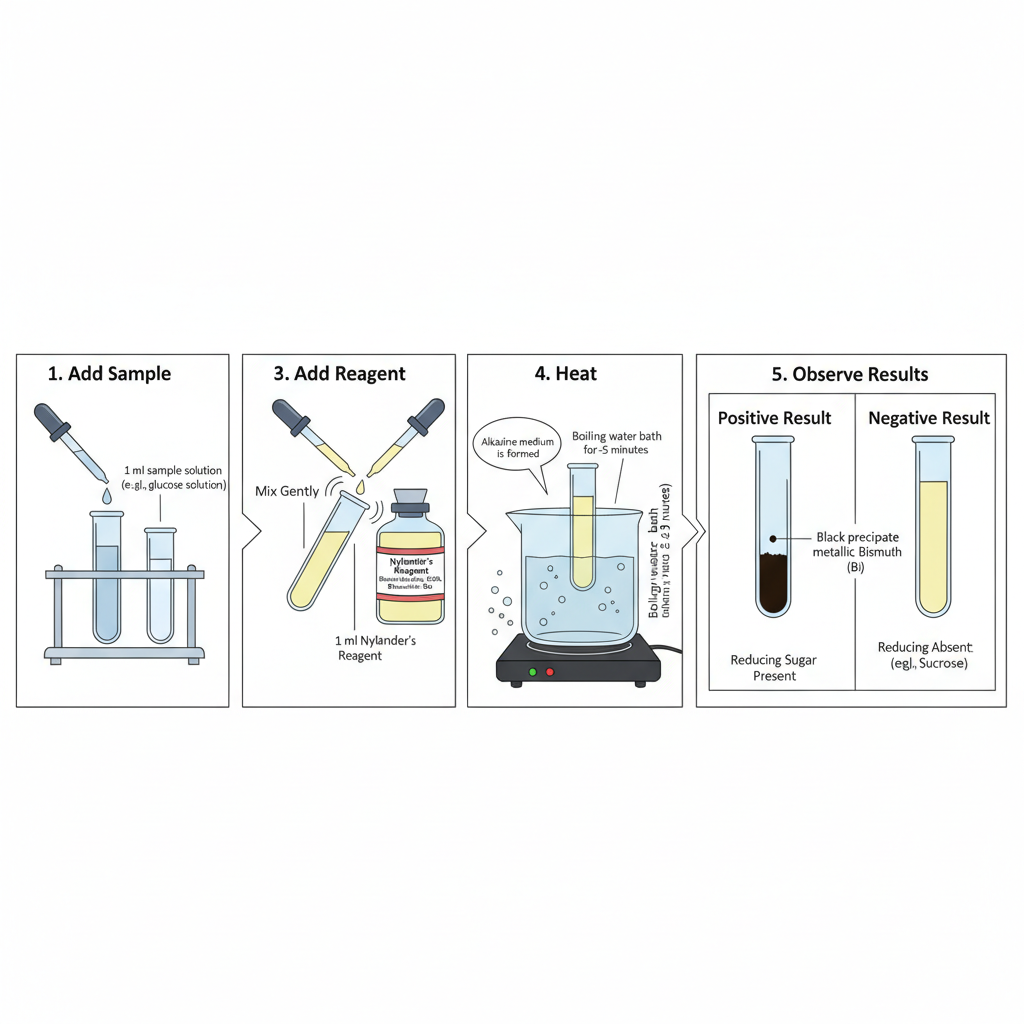
Step-by-Step Procedure of Nylander’s Test
- In this test the sample solution is first taken in a clean test tube. It is usually 1 ml of the suspected solution which may contain reducing sugar.
- An equal amount (1 ml) of freshly prepared Nylander’s reagent is added into the test tube. The reagent contain bismuth salt, alkali (KOH/NaOH) and potassium sodium tartrate which keep bismuth ions in solution.
- The mixture is shaken slightly so the components mix properly. It is the step where the alkaline medium is produced and the sugar molecules is now activated.
- The test tube is placed in a boiling water bath. Heating is essential because the reduction of bismuth subnitrate occur only at high temperature.
- The mixture is usually heated for around 2–5 minutes. In this period the reducing sugar, if present, start reducing bismuth salt into metallic bismuth.
- After heating, the test tube is removed carefully and allowed to stand. Formation of black or dark brown precipitate is seen at the bottom which is referred to as metallic bismuth.
- If the solution remain clear or only slight turbidity is seen then reducing sugar is absent. Sucrose solution is generally used as negative control since it is a non-reducing sugar.
Nylander’s Test Result
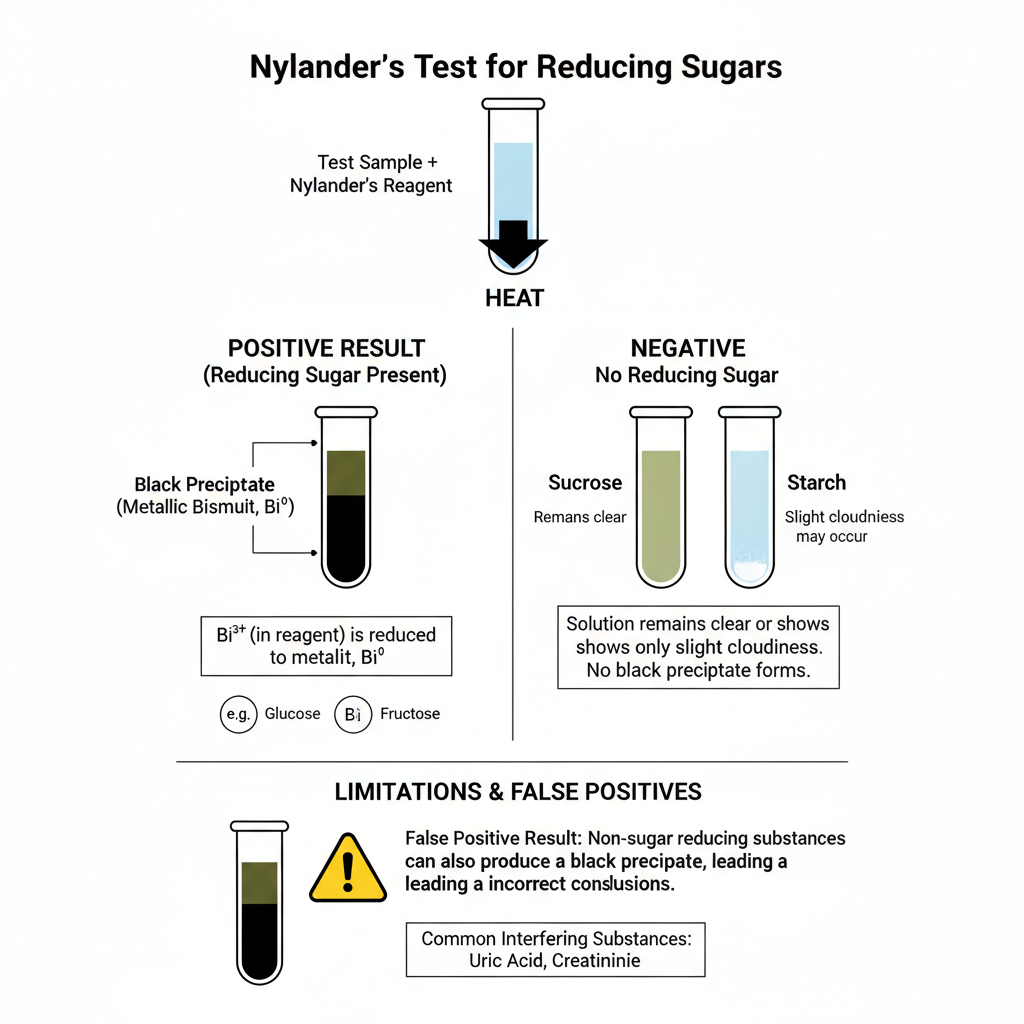
Positive Result
When reducing sugar is present the mixture after heating shows a dark brown to black colour.
It is the formation of metallic bismuth (Bi) which is produced when the bismuth salt is reduced in alkaline condition.
The black precipitate usually settles down at the bottom of the test tube and the fluid becomes darker.
Glucose solution mostly shows dark olive green to black appearance and fructose may show olive green or dark brown type colour.
The reaction is generally seen when sugar concentration is around 0.1% or more.
Negative Result
In absence of reducing sugar the solution remain clear or only slight cloudiness is observed.
Sucrose solution act as a typical negative control because it is non-reducing and remain clear olive green.
Large carbohydrates like starch or glycogen usually remain clear but starch sometimes shows slight cloudiness at the bottom.
Any dark colour that appear only during cooling is not considered as positive.
False Results and Limitations
The test is highly sensitive but it is not very specific. It is the problem because many non-sugar reducing substances also reduce bismuth salt in strong alkaline condition.
Uric acid, creatinine and similar compounds present in biological samples may give dark precipitate, leading to false positive result.
Some descriptions mentioning copper sulphate or red precipitate is not correct because Nylander’s test uses bismuth salt and the positive result is black metallic bismuth only.

Uses of Nylander’s Test
- It is used mainly for detection of reducing sugars. The test works because reducing sugars convert the bismuth salt into metallic bismuth in alkaline condition.
- Glucose, fructose, lactose and some other reducing sugars can be identified since they produce black or dark brown precipitate after heating.
- It was an important clinical test earlier for detecting glucose in urine. It is the process used for confirming glucosuria in suspected diabetes cases because sugar above around 0.1% gives positive reaction.
- In histology labs the reagent is sometimes applied for detecting reducing sugars in tissue section since the black–brown colour is easily visible under microscope.
- It is used in teaching laboratory to show the reducing property of carbohydrates and how alkaline medium and heat help in enolization of sugar.
- It also help in explaining non-specific redox type assays where sensitivity is high but specificity is not strong.
Advantages of Nylander’s Test
- It is a very sensitive test for detecting glucose. The reaction is observed even when the sugar level is around 0.1% or more, and the formation of black metallic bismuth gives a clear visible end point.
- The production of dense black precipitate of metallic bismuth (Bi) make the result easy to identify since the colour contrast is strong in alkaline medium.
- Nylander’s reagent is stable for long time. When it is prepared correctly it remain unchanged for months, so it is easier to store as single solution compared to copper-based reagents.
- Potassium sodium tartrate in the reagent keep the bismuth ions in solution and stop formation of insoluble bismuth hydroxide, so the reagent is more reliable.
- It can be used in histology for detecting reducing sugars in tissue section because the black–brown colour is easily seen under microscope.
- The reagent can detect not only monosaccharides but also some disaccharides and few polysaccharides which shows reducing property.
- In teaching laboratories it is useful to demonstrate reducing nature of carbohydrates and to show how non-specific redox type tests work in alkaline condition.
Limitations of Nylander’s Test
- The major limitation is that the test is not specific. It is the problem because many non-sugar reducing substances also reduce the bismuth salt in strong alkaline and hot condition.
- Uric acid, creatinine and other reducing agents present normally in biological samples can give the same black metallic bismuth precipitate, so false positive result is common.
- Because of this interference the test is unreliable for detecting glucose in urine since positive result cannot confirm true glucosuria.
- The test is only qualitative. It shows only presence or absence of sugar and do not give any information about sugar concentration.
- Modern enzymatic tests replaced it since they are more specific and quantitative, but Nylander’s test cannot provide accurate measurement.
- If heating is not proper or heating time is short, then reduction may not complete, and little or no precipitate is produced which may give false negative result.
- Very strong alkali or too long heating can decompose the sugar, forming other products that disturb the observation.
- Any dark colour formed only during cooling is not considered positive, so sometimes interpretation becomes difficult.
- Some descriptions confuse it with copper-based tests mentioning blue or red precipitate, which is not correct since Nylander’s test is bismuth based and only black metallic bismuth is produced.
Precautions
- The reagent must be prepared in correct order. Bismuth salt and potassium sodium tartrate is dissolved in strong alkali so the bismuth ions remain in soluble tartrate complex.
- It is important that bismuth hydroxide is not formed, so tartrate must be present properly to keep Bi ions available for reaction.
- During reagent preparation the solution is usually heated around 50°C and filtered after cooling so that the reagent stay stable for long time.
- The test mixture must always be heated because the reduction reaction occurs only under boiling condition.
- The dark colour must appear during boiling, because any colour formed on cooling is not taken as positive.
- Excess heating or too strong alkali may degrade the sugar and disturb the reaction, so heating time should be controlled.
- Since the test is not specific, substances like uric acid and creatinine can also reduce bismuth salt and give black precipitate. This must be remembered during interpretation.
- For clinical samples such as urine, a positive result cannot confirm glucose directly and follow-up tests is needed.
- It is also important to identify true Nylander’s test because it produces black metallic bismuth only. Any description mentioning red or blue colour indicate copper-based tests and not Nylander’s test.
- BYJU’S. (n.d.). Tests of carbohydrates. Retrieved from [Source URL not provided in excerpt]
- Chemistry Stack Exchange. (2025). Functional difference of Benedict’s solution and Fehling’s solution.
- Dela Cruz, G. (2023). Post-lab activity No. 12 test for carbohydrates [PDF document]. Scribd.
- Filo EdTech INC. (2025). nylander’s test for carbohydrates structures. Filo.
- Food Safety and Standards Authority of India. (2015). Manual of methods of analysis of foods milk and milk products.
- Food Safety and Standards Authority of India. (2016). Manual of methods of analysis of foods: Milk and milk products.
- Hernández-López, A., Sánchez Félix, D. A., Z. Z.-S., I. G.-B., T. D. D., & A. X. A.-A. (2020). Quantification of reducing sugars based on the qualitative technique of Benedict. ACS Omega, 5(50), 32403–32410. doi: 10.1021/acsomega.0c04467
- Lumongsud, M. A. C. (n.d.). Biochem lab tests explained | PDF | materials | atoms [PDF document]. Scribd. Retrieved from
- Morphisto. (n.d.). NYLANDER’s Reagenz. Retrieved from
- Nylander’s test. (2023). In Wikipedia. Retrieved from
- Nylander’s test. (2024). In Wikipedia. Retrieved from
- Nylanders test [PDF document]. (n.d.). Scribd. Retrieved from
- ResearchGate. (2022). (PDF) The mechanism of Nylander’s test for glucose in urine. Retrieved from [Source URL not provided in excerpt]
- The Nylander’s assay: Comprehensive review of the bismuth reduction principle for reducing sugar detection, mechanism, and analytical performance. (n.d.). Retrieved from [Source URL not provided in excerpt]
- preparation of special analytical reagents. (n.d.). In CRC handbook of chemistry and physics (91st ed.). CRC Press.
- Glucose. (2024). In Wikipedia. Retrieved from [Source URL not provided in excerpt]
- Glucose in urine test: MedlinePlus medical test. (2024). U.S. National Library of Medicine. Retrieved from
- Siemens Healthcare Diagnostics, Inc. (2016). 510(k) substantial equivalence determination decision memorandum assay only template (K161494). U.S. Food and DrugAdministration.Retrieved from
- Soriano, A. G. (n.d.). Post lab finals3 | PDF | glucose | starch [Presentation]. Scribd. Retrieved from
- Testbook. (n.d.). Fehling test – reaction, reagent, mechanism, and difference between Fehling’s and Benedict’s test. Retrieved from
- Thomas, L. (n.d.). Chapter 53: Laboratory organization. In Clinical laboratory diagnostics.: TH-Books.
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.