What is Nucleoside?
A nucleoside is a molecule composed of a pentose sugar linked to a nitrogenous base or glycosylamine. It can be considered as a nucleotide without a phosphate group. Nucleosides are essential components of DNA and RNA, playing a vital role in the storage and transmission of genetic information.
In DNA, the nucleosides contain a 2′-deoxy-D-ribose sugar, while in RNA, the nucleosides contain D-ribose sugar. The key distinction lies in the second position of the pentose structure. In 2′-deoxyribose, there is an absence of an alcohol group (-OH) at the second position, leading to the name “2′-deoxy.” On the other hand, D-ribose has an -OH group present at the second position. Both types of pentoses exist in their β-furanose form, which is a close five-membered ring structure.
Nucleosides are considered glycosylamines since they consist of a nucleobase (also known as a nitrogenous base) and a five-carbon sugar (ribose or 2′-deoxyribose). The nucleobase can be a purine (such as adenine or guanine) or a pyrimidine (such as cytosine, thymine, or uracil). In a nucleoside, the anomeric carbon of the sugar molecule is linked through a glycosidic bond to the N9 position of a purine base or the N1 position of a pyrimidine base.
In contrast, nucleotides are more complex molecules that contain a nucleobase, a five-carbon sugar, and one or more phosphate groups. The phosphate groups in nucleotides play crucial roles in energy transfer, signaling pathways, and enzymatic reactions.
Overall, nucleosides serve as the fundamental units from which nucleotides are derived. They are involved in DNA and RNA synthesis and function as intermediates in various metabolic pathways. Understanding the structure and properties of nucleosides is essential for comprehending the molecular processes that underlie genetics and biochemistry.
Definition of Nucleoside
A nucleoside is a type of organic molecule consisting of a nitrogenous base (also known as a nucleobase) and a sugar molecule. The sugar in a nucleoside can be either ribose or deoxyribose, and it is linked to the nitrogenous base through a glycosidic bond.
Nucleosides are fundamental building blocks of nucleotides, which are the monomers that make up nucleic acids like DNA and RNA. A nucleotide consists of a nucleoside combined with one or more phosphate groups. The phosphate groups are responsible for forming the backbone of DNA and RNA and are crucial for their structural stability and function.
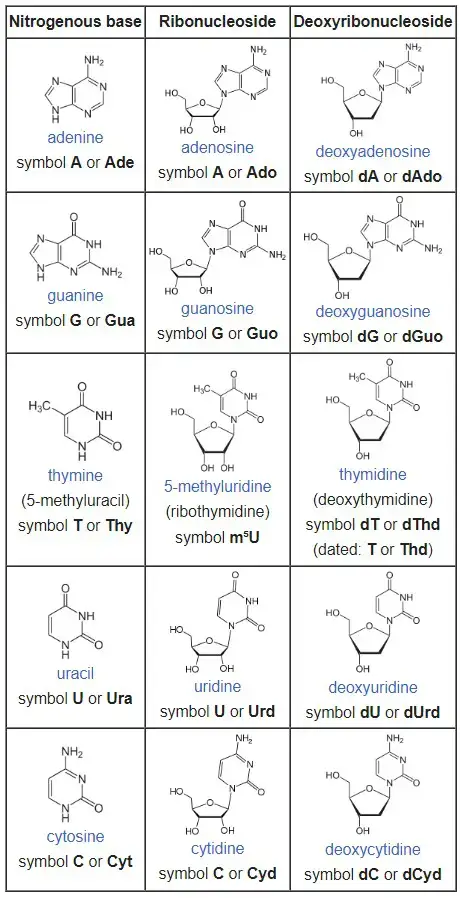
The nitrogenous base in a nucleoside can be one of several types, including adenine (A), guanine (G), cytosine (C), thymine (T), and uracil (U). The specific combination of the nitrogenous base and the sugar molecule determines the type of nucleoside. For example, if a nucleoside contains the nitrogenous base adenine and the sugar ribose, it is called adenosine.
Nucleosides play important roles in cellular processes. They are involved in DNA replication, transcription, and translation, which are essential for the storage and expression of genetic information. Nucleosides can also have additional functions in cellular signaling and metabolism.
In summary, a nucleoside is a molecule composed of a nitrogenous base and a sugar molecule, and it serves as a foundational unit for the construction of nucleotides and nucleic acids.
Characteristics of Nucleoside
- Composition: A nucleoside consists of two main components:
- Nucleobase: It can be a purine (such as adenine or guanine) or a pyrimidine (such as cytosine, thymine, or uracil) nucleobase.
- Pentose Sugar: The nucleobase is attached to a pentose sugar component, which can be either ribose (in RNA) or deoxyribose (in DNA).
- Structure: In a nucleoside, the nucleobase is linked to the pentose sugar through a glycosidic bond. The anomeric carbon of the sugar is attached to the N9 of a purine nucleobase or the N1 of a pyrimidine nucleobase.
- Formation: Nucleosides are formed through the hydrolysis of nucleic acids. When a nucleic acid is broken down, the covalent bonds between the nucleobases and the sugar components are cleaved, resulting in the formation of nucleosides.
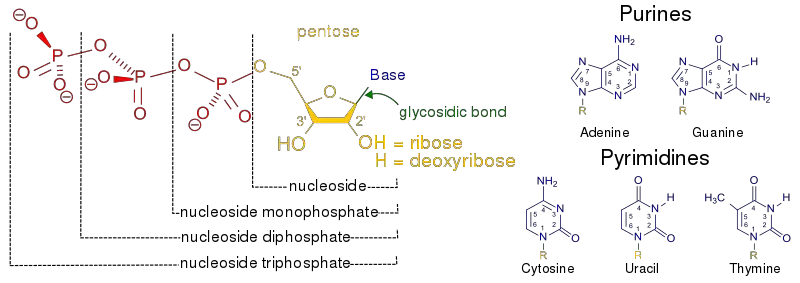
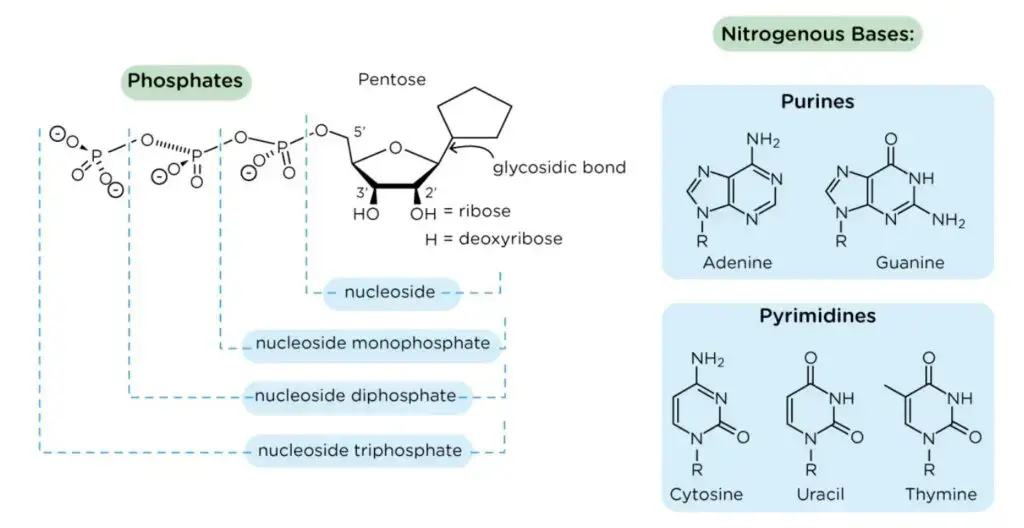
- Relationship with Nucleotides: Nucleosides serve as the building blocks for nucleotides. When a phosphate group is covalently attached to the pentose sugar of a nucleoside, it forms a nucleotide. The addition of the phosphate group to the nucleoside provides the energy and chemical stability necessary for nucleotides to fulfill their various functions in cellular processes.
- Role: Nucleosides have important roles in cellular metabolism and signaling. They can act as intermediates in nucleotide synthesis, participating in the formation of DNA and RNA. Additionally, nucleosides can serve as signaling molecules and be involved in various biochemical pathways within the cell.
Types of Nucleoside Based on nucleobase component
Based on the presence of the nitrogen base of the compound, the nucleosides can be divided into two distinct categories.
- Purine nucleosides
- Pyrimidine nucleosides
Purine nucleosides
- Purine nucleosides are a class of nucleosides that are composed of two specific nitrogenous bases: adenine and guanine. These nucleosides play important roles in the structure and function of nucleic acids, specifically RNA and DNA.
- In RNA, the two purine nucleosides present are adenine and guanine. Adenine forms the nucleoside adenosine, while guanine forms the nucleoside guanosine. These nucleosides are essential components of RNA molecules and contribute to the genetic code and protein synthesis.
- In DNA, the purine nucleosides are slightly different due to the presence of deoxyribose, a modified sugar molecule. The purine nucleoside derived from adenine is called deoxyadenosine, and the purine nucleoside derived from guanine is called deoxyguanosine. These nucleosides, along with the other nucleosides derived from the pyrimidine bases (cytosine, thymine, and uracil), form the building blocks of DNA. They are involved in the formation of complementary base pairs, which stabilize the DNA double helix structure and facilitate DNA replication and transmission of genetic information.
- Purine nucleosides are crucial for cellular processes such as DNA replication, transcription, and translation. They contribute to the overall stability and integrity of nucleic acids and play a vital role in genetic information storage and expression.
- In summary, purine nucleosides are nucleosides composed of adenine and guanine. They are important components of RNA and DNA, serving as building blocks for nucleic acids and participating in various cellular processes essential for genetic information transfer and protein synthesis.
Pyrimidine nucleosides
- Pyrimidine nucleosides are a class of nucleosides that consist of three specific nitrogenous bases: thymine, cytosine, and uracil. These nucleosides play essential roles in the structure and function of nucleic acids, specifically RNA and DNA.
- In RNA, the two pyrimidine nucleosides present are cytosine and uracil. Cytosine forms the nucleoside cytidine, while uracil forms the nucleoside uridine. These nucleosides are integral components of RNA molecules and are involved in various cellular processes, including protein synthesis and regulation of gene expression.
- In DNA, the pyrimidine nucleosides are slightly modified due to the presence of deoxyribose, a modified sugar molecule. The pyrimidine nucleoside derived from cytosine is called deoxycytidine, and the pyrimidine nucleoside derived from thymine is called deoxythymidine or thymidine. These nucleosides, along with the purine nucleosides (adenosine and guanosine), form the building blocks of DNA. They contribute to the formation of complementary base pairs, which stabilize the DNA double helix structure and ensure accurate DNA replication and transmission of genetic information.
- Pyrimidine nucleosides are involved in critical cellular processes such as DNA replication, repair, and transcription. They are also crucial for the regulation of gene expression and the production of functional RNA molecules.
- In summary, pyrimidine nucleosides consist of thymine, cytosine, and uracil. They are vital components of RNA and DNA, serving as the building blocks for nucleic acids and participating in various cellular processes necessary for genetic information storage, regulation, and expression.
Types of Nucleoside Based on pentose sugar
Here are the types of nucleosides based on the pentose sugar component:
- Ribonucleosides:
- Ribonucleosides have a ribose sugar component.
- Adenosine: The nucleobase component is adenine.
- Guanosine: The nucleobase component is guanine.
- Cytidine: The nucleobase component is cytidine.
- Uridine (or 5-methyluridine): The nucleobase component is uracil.
- Deoxyribonucleosides:
- Deoxyribonucleosides have a deoxyribose sugar component.
- Deoxyadenosine: The nucleobase component is adenine.
- Deoxyguanosine: The nucleobase component is guanine.
- Deoxycytidine: The nucleobase component is cytidine.
- Thymidine (or deoxyuridine): The nucleobase component is uracil.
These different types of nucleosides, distinguished by their pentose sugar component, play crucial roles in nucleic acid structures. Ribonucleosides are integral to RNA molecules, while deoxyribonucleosides are key components of DNA molecules. The specific combination of the nucleobase and pentose sugar in each nucleoside contributes to the diversity and functionality of nucleic acids in various cellular processes.
Structure of Nucleoside
The nucleosides consist of two main heterocyclic components –
Pentose sugar
- The pentose sugar is an essential component of nucleosides and nucleotides, which are building blocks of nucleic acids like DNA and RNA. It is a five-membered ring structure that exhibits a puckered conformation.
- In the case of DNA, the pentose sugar is called 2′-deoxy-D-ribose. It differs from the pentose sugar in RNA by the absence of an -OH group at the second position of the ring. This modification gives rise to the name “2′-deoxy,” indicating the lack of an oxygen group. In RNA, the pentose sugar is referred to as D-ribose, and it contains an -OH group at the second position.
- Both DNA and RNA pentose sugars adopt the β-furanose form, which is a close, five-membered ring structure. This conformation is stable and facilitates the formation of nucleosides and nucleotides.
- The pentose sugar is attached to the nitrogenous base of a nucleoside at the primary carbon atom, also known as the 1′ carbon. This linkage occurs through a glycosidic bond, specifically an N-β-glycosyl bond. The glycosidic bond connects the sugar and the nitrogenous base, forming the nucleoside.
- The structure of the pentose sugar is crucial for the stability and function of nucleic acids. It provides a backbone to which the nitrogenous bases are attached, forming the genetic code and allowing for the storage and transmission of genetic information.
Nitrogenous base
- A nitrogenous base is a cyclic carbon structure containing nitrogen and exhibiting basic properties. These bases are integral components of nucleotides, which are the building blocks of nucleic acids such as DNA and RNA.
- There are two major types of nitrogenous bases: purines and pyrimidines. In RNA, the nitrogenous bases include adenine, uracil, cytosine, and guanine. In DNA, the nitrogenous bases are adenine, thymine, cytosine, and guanine. These bases differ slightly between RNA and DNA, with uracil replacing thymine in RNA.
- The nitrogenous bases are covalently bonded to the pentose sugar of nucleotides through an N-β-glycosyl bond. In purines, the N-9 atom forms this bond with the pentose sugar, while in pyrimidines, the N-1 atom is involved in the bonding.
- Although the majority of bases are derived from purines and pyrimidines, there are also minor bases present in DNA. These minor bases often occur in methylated forms of purines and pyrimidines. In some cases, the bases in viral DNA may be hydroxyl-methylated or glycosylated, introducing further variations.
- Some examples of unusual bases found in DNA include 5-methylcytidine, N6-methyladenosine, 7-methylguanosine, and 4-thiouridine. These bases may have specific roles in DNA structure, function, or regulation, and they contribute to the diversity and complexity of genetic information.
Functions of Nucleosides
Nucleosides serve various important functions in biological systems. Here are some key roles of nucleosides:
- Precursors for Nucleotides: Nucleosides are building blocks for nucleotides, which are essential for the synthesis of DNA and RNA. When a phosphate group is attached to a nucleoside, it forms a nucleotide, serving as the backbone of DNA and RNA molecules.
- Signaling Molecules: Certain nucleosides, such as adenosine, function as signaling molecules in cellular communication. Adenosine, for example, plays a role in regulating various physiological processes including neurotransmission, immune response, and vascular tone.
- Regulation and Protection of Genetic Information: Minor bases or altered nitrogen bases found in nucleosides can have regulatory functions in gene expression and protect genetic information. These modified nucleosides can affect the structure, stability, and processing of RNA molecules.
- Therapeutic Applications: Nucleoside analogs, which are modified versions of nucleosides, have been developed and used in the treatment of malignancies, tumors, and viral infections. These analogs, by incorporating into DNA or RNA during replication or transcription, disrupt the normal processes and inhibit the growth of cancer cells or viral replication. For example, cytarabine (cytosine arabinoside) is a nucleoside analog approved for treating acute myeloid leukemia, and nucleoside analogs like lamivudine are used in the treatment of HIV.
- Nucleoside Transport: Nucleoside transporters are proteins that play a crucial role in the transportation of nucleosides across cell membranes. There are two types of nucleoside transporters: concentrative and equilibrative transporters. These transporters are involved in the uptake and efflux of nucleosides, including nucleoside analogs used in antiviral and anticancer therapies.
Overall, nucleosides are not only essential components of DNA and RNA but also have diverse roles as signaling molecules, regulators of genetic information, and therapeutic agents in medical treatments.
Examples of Nucleosides
Nucleosides are organic compounds composed of a nitrogenous base linked to a sugar molecule. They are important building blocks of nucleotides, which are the basic units of nucleic acids such as DNA and RNA. Here are some examples of nucleosides:
- Adenosine: Adenosine is a purine nucleoside that consists of adenine bound to a ribose sugar by a glycosidic bond. It is found in DNA, RNA, and various molecules involved in cellular energy transfer, such as ATP, ADP, and AMP. Adenosine also acts as a neurotransmitter and is involved in processes like sleep regulation.
- Guanosine: Guanosine is another purine nucleoside composed of guanine attached to a ribose sugar. It can be converted into different nucleotides, including guanosine monophosphate (GMP), cyclic guanosine monophosphate (cGMP), guanosine diphosphate (GDP), and guanosine triphosphate (GTP). These nucleotides play crucial roles in biochemical processes like nucleic acid synthesis, protein synthesis, and intracellular signaling.
- Cytidine: Cytidine is a pyrimidine nucleoside that contains cytosine linked to a ribose sugar. It has been suggested to have potential antidepressant effects and may regulate neuronal-glial glutamate cycling.
- Uridine: Uridine is a ribonucleoside comprising uracil attached to a ribose ring. It is involved in carbohydrate metabolism and plays a role in various cellular processes.
- 5-methyluridine: 5-methyluridine is a pyrimidine nucleoside with a thymine nucleobase connected to a ribose sugar. It exists as a small white crystalline solid.
- Deoxyadenosine: Deoxyadenosine is a purine nucleoside similar to adenosine, but with a deoxyribose sugar instead of ribose. It is a component of DNA and differs from adenosine in its sugar moiety.
- Deoxyguanosine: Deoxyguanosine is a purine nucleoside that consists of guanine attached to a deoxyribose sugar. It is a component of DNA and differs from guanosine by its sugar component.
- Deoxycytidine: Deoxycytidine is a pyrimidine nucleoside that contains cytosine attached to a deoxyribose ring. It is similar to cytidine but lacks one oxygen atom.
- Thymidine: Thymidine is a pyrimidine nucleoside that has thymine bound to a deoxyribose sugar. It is primarily found in DNA and is involved in DNA synthesis. The prefix “deoxy-” is often omitted when referring to thymidine since it is exclusive to DNA synthesis.
- Deoxyuridine: Deoxyuridine is a deoxyribonucleoside with uracil attached to a deoxyribose ring. It acts as an antimetabolite and can interfere with DNA synthesis when incorporated into DNA during replication.
- Inosine: Inosine is a nucleoside formed when hypoxanthine is linked to a ribose ring through a β-N9-glycosidic bond. It is commonly found in transfer RNAs (tRNAs) and is involved in purine nucleotide reactions during muscle movements.
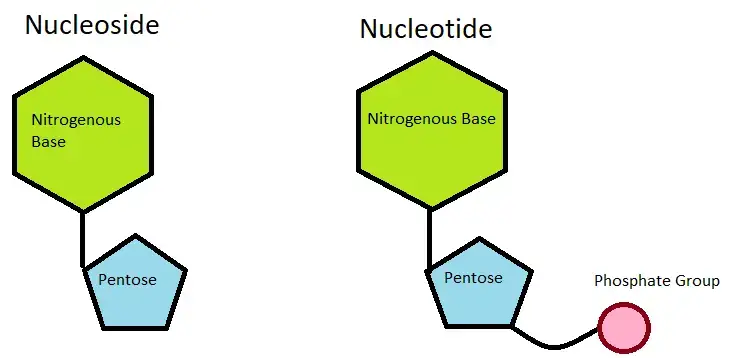
Difference between nucleotide and nucleoside – Nucleotides vs Nucleosides
| Characteristics | Nucleotides | Nucleosides |
|---|---|---|
| Definition | Building blocks of nucleic acids | Molecules composed of a nucleobase and a sugar molecule |
| Composition | Nitrogenous base + Sugar + Phosphate | Nitrogenous base + Sugar |
| Components | Nitrogenous base (adenine, guanine, cytosine, thymine/uracil) + Sugar (ribose/deoxyribose) + Phosphate group | Nitrogenous base + Sugar (ribose/deoxyribose) |
| Examples | Adenosine triphosphate (ATP), guanosine triphosphate (GTP), cytidine triphosphate (CTP), thymidine triphosphate (TTP/UTP) | Adenosine, guanosine, cytidine, uridine, thymidine |
| Function | Serve as the building blocks for DNA and RNA, energy carriers (ATP), signaling molecules (cyclic AMP) | Act as intermediates in nucleotide synthesis, play roles in cellular metabolism and signaling |
| Phosphate Group | Contains one or more phosphate groups attached to the sugar | Does not contain phosphate groups |
| Importance | Essential for genetic information storage, transfer, and expression | Play important roles in cellular processes and signaling |
FAQ
What is a nucleoside?
A nucleoside is an organic compound consisting of a nitrogenous base (either a purine or a pyrimidine) attached to a sugar molecule (ribose or deoxyribose) through a glycosidic bond.
How are nucleosides different from nucleotides?
Nucleosides are composed of a nitrogenous base and a sugar, while nucleotides have an additional phosphate group attached to the sugar. Nucleotides are considered the building blocks of nucleic acids (DNA and RNA), whereas nucleosides are important components of nucleotides.
What are the functions of nucleosides in the body?
Nucleosides play crucial roles in various biochemical processes. They are involved in DNA and RNA synthesis, energy transfer (e.g., ATP), intracellular signaling, protein synthesis, and neurotransmission.
Where are nucleosides found?
Nucleosides are found in all living organisms as essential components of DNA, RNA, and various molecules involved in cellular processes. They can also be synthesized and used as therapeutic agents in medicine.
Can nucleosides be obtained from dietary sources?
Yes, nucleosides can be obtained from dietary sources. For example, foods rich in nucleosides include meat, fish, legumes, and some vegetables. However, most nucleosides in the body are synthesized de novo or recycled from nucleic acid breakdown.
Are nucleosides used in medicine?
Yes, nucleosides and their derivatives are commonly used in medicine. They can be used as antiviral agents, anticancer drugs, and treatments for other diseases. Some nucleoside analogs can inhibit viral replication or interfere with DNA synthesis in cancer cells.
Are nucleosides involved in genetic disorders?
Yes, genetic disorders can arise from mutations or deficiencies in enzymes responsible for nucleoside metabolism. These disorders, such as Lesch-Nyhan syndrome or mitochondrial diseases, can lead to various symptoms and health complications.
Can nucleosides be synthesized in the laboratory?
Yes, nucleosides can be chemically synthesized in the laboratory. Synthetic nucleosides have diverse applications in research, drug development, and other fields of biotechnology.
Can nucleosides be modified or engineered?
Yes, nucleosides can be modified or engineered to create new compounds with specific properties. This field of research, known as nucleoside engineering, aims to develop nucleoside analogs with improved therapeutic efficacy or novel functions.
Are there any side effects or risks associated with nucleoside-based medications?
Like any medication, nucleoside-based drugs can have side effects. These may vary depending on the specific drug and its target. Common side effects include gastrointestinal disturbances, allergic reactions, and, in some cases, bone marrow suppression. It is important to consult with a healthcare professional for guidance and monitoring when using nucleoside-based medications.
References
- Mikhailopulo IA, Miroshnikov AI. New trends in nucleoside biotechnology. Acta Naturae. 2010 Jul;2(2):36-59. PMID: 22649640; PMCID: PMC3347554.
- https://www.ebi.ac.uk/chebi/searchId.do?chebiId=33838
- https://www.britannica.com/science/dietary-supplement
- https://www.sciencedirect.com/topics/medicine-and-dentistry/nucleoside
- https://accessmedicine.mhmedical.com/content.aspx?bookid=2355§ionid=185844443
- https://pubs.acs.org/doi/10.1021/cr5002035