What is the Ninhydrin Test?
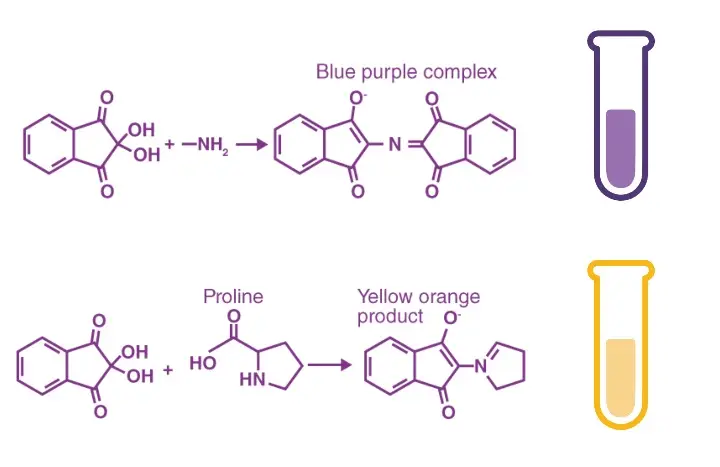
It is the chemical test used for detecting amino acids and other compounds having free amino groups. It is the process in which ninhydrin (2,2-dihydroxyindane-1,3-dione) reacts with amino acids and the reaction is accompanied by oxidative deamination. In this step, the amino group is removed and there is release of ammonia, carbon dioxide, and an aldehyde. This ammonia then reacts with ninhydrin to form a deep blue or purple coloured complex which is referred to as Ruhemann’s purple. The colour intensity is proportional to the concentration of amino acids present in the solution. It is observed that most amino acids produce this typical purple colour while compounds such as proline give a yellow colour because they possess secondary amino groups.
It is an important test used in biochemical analysis. The major source of its application is in detecting latent fingerprints on porous surfaces. It is also used in studying protein samples and in monitoring peptide synthesis reactions.
Objectives of Ninhydrin Test
- To detect the presence of free amino groups in amino acids, peptides, proteins, and amines.
- To estimate the concentration of amino acids as the colour intensity is proportional to their amount.
- To visualise latent fingerprints on porous surfaces by reacting with amino acids present in sweat residues.
- To check the progress of peptide synthesis by confirming coupling and deprotection steps.
- To locate amino acid spots after separation in paper chromatography or TLC.
- To differentiate amino acids from carbohydrates and distinguish primary amines forming purple colour from secondary amines forming yellow colour.
- To assist in clinical tests for metabolic disorders by identifying amino acids in body fluids.
- To evaluate protein content in food samples and check the purity of pharmaceutical preparations.
Principle of Ninhydrin Test
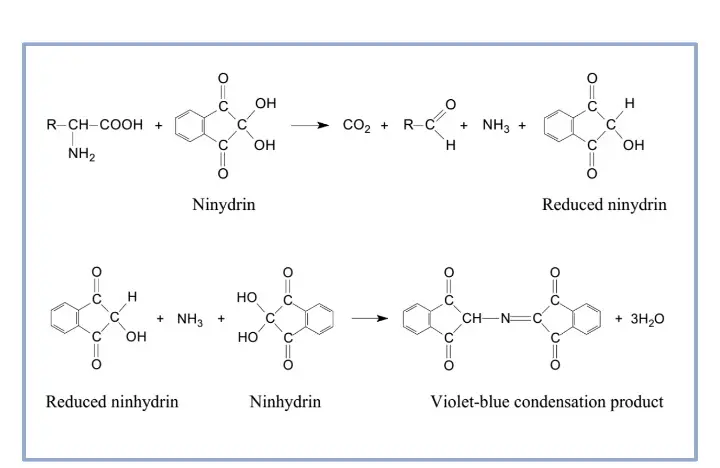
It is the principle based on the reaction between ninhydrin and the free α-amino group present in amino acids, peptides, and proteins. In this reaction, ninhydrin acts as a strong oxidizing agent and at higher temperature it causes oxidative deamination and decarboxylation of the amino acid. The amino acid is broken down releasing carbon dioxide (CO₂), an aldehyde having one carbon less than the parent amino acid, and free ammonia (NH₃). During this reaction, ninhydrin itself is reduced to hydrindantin. The released ammonia now reacts with one molecule of hydrindantin and another molecule of ninhydrin forming the coloured product which is referred to as Ruhemann’s purple. It is the deep violet complex that shows maximum absorption at 570 nm and its intensity indicates the amount of free amino groups present in the sample.
It is also noted that imino acids such as proline do not follow the same colour reaction. As these compounds lack a free primary amino group, the reaction forms a yellow to orange coloured iminium salt instead of the purple complex. The absorption of this compound is around 440 nm and thus it is measured differently.
Ninhydrin Test Reaction
Requirements
- Ninhydrin solution (generally prepared as 0.2%–2% in suitable solvent).
- Solvents like ethanol, acetone, or n-butanol are used for dissolving ninhydrin.
- Buffer solutions such as acetate or citrate buffer are required to maintain proper pH during quantitative tests.
- Reducing agents like hydrindantin or stannous chloride may be used for stabilising the reaction.
- For Kaiser test, phenol, pyridine, and potassium cyanide (KCN) are required.
- Test tubes, stand, and other vessels needed for conducting the reaction.
- Heating source such as a boiling water bath or heating block to maintain 90–100°C.
- Pipettes or droppers for transferring the reagents and samples.
- Spectrophotometer or colorimeter for measuring absorbance at specific wavelengths.
- Centrifuge may be used for removing insoluble particles if required.
- Test sample containing amino acids, peptides, or proteins.
- Standard amino acid solution for comparison and calibration.
- Negative control such as distilled water.
- Filter paper or chromatographic plates when used for fingerprint detection or chromatographic studies.
- Protective equipment like gloves, goggles, and apron because ninhydrin stains and is irritant.
- Work should be done under good ventilation, preferably inside a fume hood when using toxic solvents.
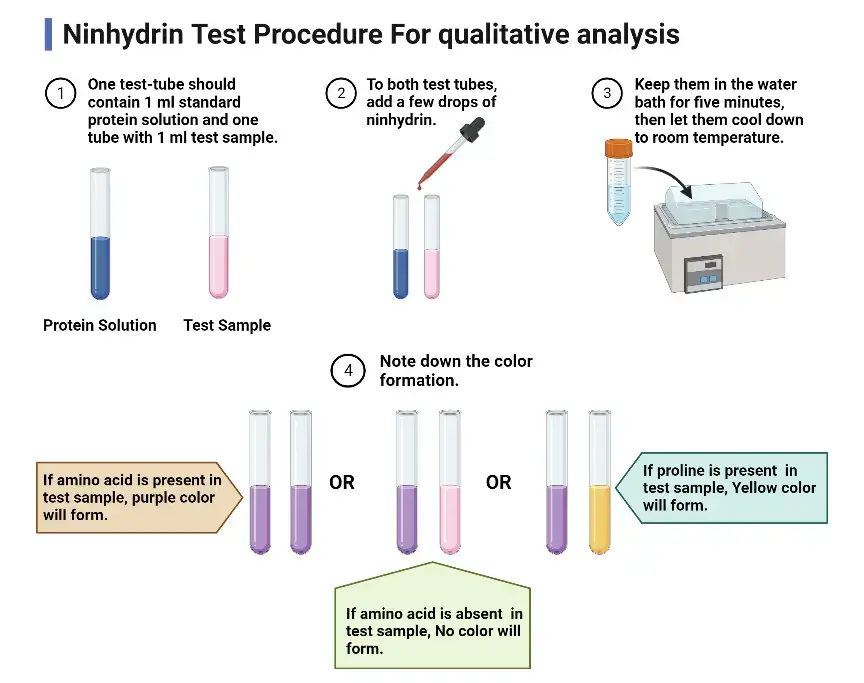
Procedure of Ninhydrin Test
- A 1% solution of the test sample is prepared in distilled water. The ninhydrin reagent is prepared by dissolving ninhydrin (0.2–2%) in ethanol or acetone or n-butanol.
- About 1–2 mL of the sample solution is taken in a clean and dry test tube. Few drops to 1 mL of ninhydrin reagent is added to it.
- The contents of the test tube is mixed properly. The tube is then placed in a boiling water bath or heating block maintained at 90–100°C.
- The mixture is heated for about 5–20 minutes. In this step the reaction is allowed to proceed completely.
- After heating the test tube is removed from the water bath and allowed to cool to room temperature.
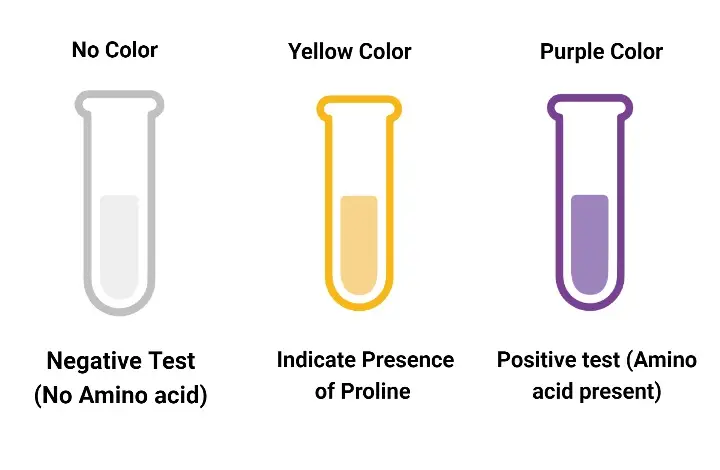
- The solution is observed for colour development. Appearance of deep blue or violet colour indicates presence of primary amino acids while yellow or orange colour indicates secondary amines (proline).
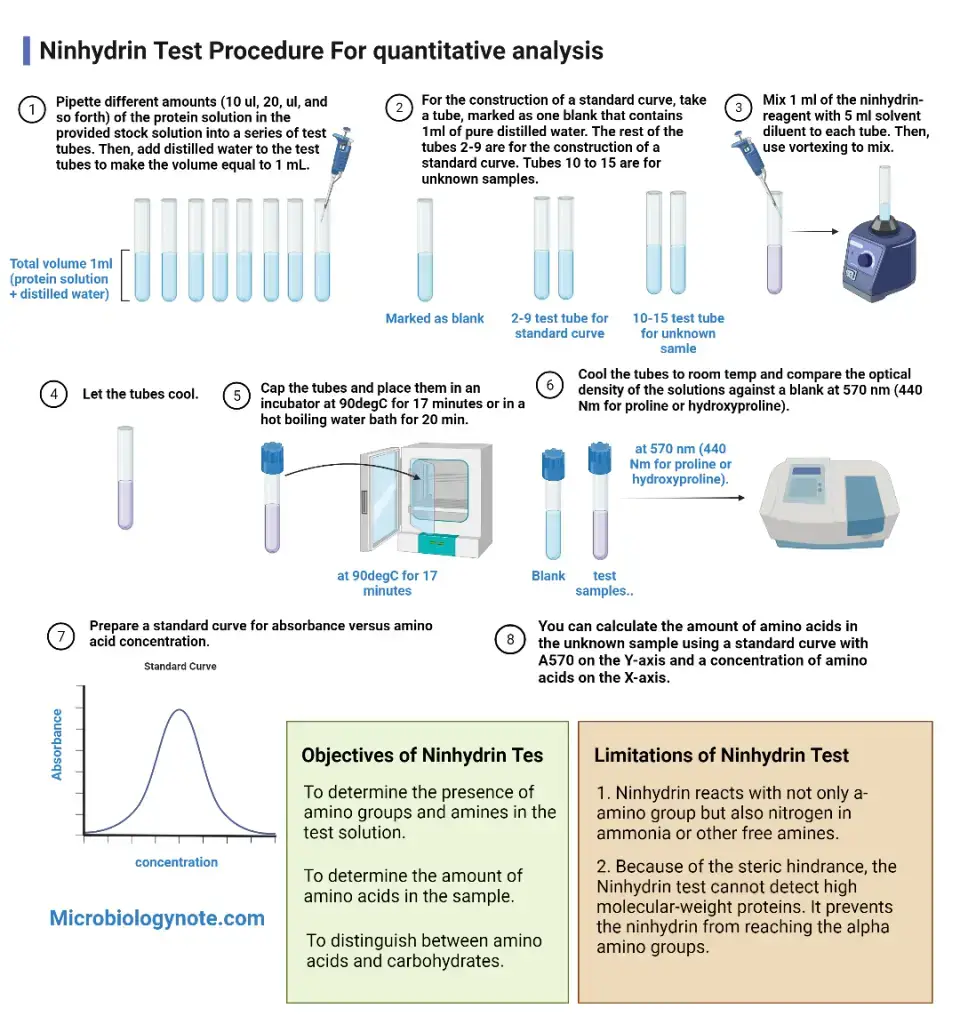
- For quantitative estimation a suitable diluent such as ethanol or water–n-propanol mixture is added and absorbance is measured at 570 nm (or 440 nm for proline).
Result of Ninhydrin Test
- Positive result (Primary amines / α-amino acids)
Deep blue or violet colour is produced. This colour is referred to as Ruhemann’s purple. It indicates the presence of primary amines, α-amino acids, peptides, proteins or ammonia. - Result for secondary amines (Imino acids)
Secondary amines such as proline and hydroxyproline give yellow or orange colour instead of purple. - Result for asparagine
Asparagine generally gives a brown coloured product after reaction with ninhydrin. - Negative result
No colour change is observed and the solution remains colourless or pale yellow. It indicates absence of free amino acids, amines or ammonia. - Forensic fingerprint analysis
When applied on porous surfaces like paper or cardboard, latent fingerprints appear as purple coloured spots or prints due to reaction with amino acids present in sweat. - Solid phase peptide synthesis (Kaiser test)
– Incomplete coupling: Resin beads and solution turn dark blue showing presence of free primary amines.
– Complete coupling: Resin beads remain colourless or light yellow indicating absence of free primary amines.
Uses of Ninhydrin Test
- It is used for detection of latent fingerprints on porous surfaces such as paper, cardboard, currency notes and unfinished wood in forensic science.
- It is used for qualitative detection and quantitative estimation of free amino acids, peptides and proteins in biological and chemical samples.
- It is used in paper chromatography and thin layer chromatography to locate amino acid spots after separation.
- It is used in solid phase peptide synthesis as Kaiser test to check completeness of amino acid coupling and deprotection steps.
- It is used in clinical diagnosis to screen metabolic disorders such as phenylketonuria (PKU) and other aminoacidurias.
- It is used in food analysis to estimate protein content, monitor cheese ripening and to measure α-amino nitrogen in food products.
- It is used to check cleanliness of medical instruments by detecting residual protein contamination after washing.
- It is used in pharmaceutical analysis to test purity of amino acids and to detect ammonium ion contamination.
- It is used in environmental and archaeological studies to detect nitrogen release in soil and to analyse ancient bone collagen.
Limitations of Ninhydrin Test
- It is not specific only for amino acids. Ninhydrin also reacts with ammonia, urea and other free amines which may give false positive result.
- It cannot differentiate between individual primary amino acids as most of them produce same purple coloured compound.
- It is less effective for detection of large intact proteins. The folded structure of high molecular weight proteins hides the α-amino groups.
- The sensitivity is lower as compared to fluorometric methods. Very low or trace amount of amino acids may not be detected accurately.
- The test is affected by contamination. Presence of ammonia in water or glassware can interfere and give high background colour.
- Ninhydrin reagent is unstable in nature. It is sensitive to light and air and degrades on long storage, so fresh reagent is often required.
- Secondary amines such as proline and hydroxyproline do not give purple colour. Asparagine may give brown colour which complicates interpretation.
- The reaction is destructive to the sample. The original material cannot be recovered after performing the test.
- Certain drugs and chemicals may interfere in clinical analysis and produce false peaks or abnormal results.
- In forensic use on thermal paper the solvent may blacken the surface and obscure fingerprint details.
Advantages of Ninhydrin Test
- It is a highly sensitive test. Very small or microgram quantity of amino acids, peptides and proteins can be detected.
- The colour intensity produced is proportional to concentration of amino acids. This helps in quantitative estimation using spectrophotometer.
- Almost all primary amino acids give same purple coloured product. This makes estimation of total amino nitrogen easy.
- It helps in differentiation between primary and secondary amines. Primary amines give blue or purple colour while secondary amines give yellow or orange colour.
- It is very effective in forensic science. It is widely used for development of latent fingerprints on porous surfaces.
- The procedure is simple and easy to perform. It does not require complex instruments or techniques.
- The test is economical in nature. Reagents and equipment used are inexpensive.
- The coloured product formed is soluble. It can be easily extracted and measured after reaction.
FAQ
- AAPPTec. (n.d.). Monitoring of peptide coupling and capping; Coupling tests. https://www.peptide.com/resources/solid-phase-peptide-synthesis/monitoring-of-peptide-coupling-and-capping/
- AAPPTec. (n.d.). Technical support information bulletin 1188: Kaiser test (Ninhydrin test). https://www.peptide.com/custdocs/1188%20ninhydrin%20test.pdf
- Alkhudair, E. M. (n.d.). Lab (2) Quantitative amino acids estimation by Ninhydrin method [PowerPoint slides]. King Saud University. https://faculty.ksu.edu.sa/sites/default/files/Lab%202_1.pdf
- Bellevue College. (n.d.). Beer’s law: Determining the concentration of a solution. https://www.bellevuecollege.edu/wp-content/uploads/sites/140/2014/06/161lab_Beers-Law-updated-1-2-2014.pdf
- BenchChem. (2025, December). Application note & protocol: Quantitative analysis of amino acids using the ninhydrin method. https://www.benchchem.com/pdf/Application_Note_Protocol_Quantitative_Analysis_of_Amino_Acids_using_the_Ninhydrin_Method.pdf
- BenchChem. (2025, December). Ninhydrin reaction optimization: Technical support center. https://www.benchchem.com/pdf/Ninhydrin_Reaction_Optimization_Technical_Support_Center.pdf
- BenchChem. (2025, December). Reducing background color in ninhydrin-based assays. https://www.benchchem.com/pdf/Reducing_background_color_in_Ninhydrin_based_assays.pdf
- Biochemistry Den. (2025, February 20). Ninhydrin test- definition, principle, procedure, result, uses. https://biochemden.com/ninhydrin-test/
- Bottom, C. B., Hanna, S. S., & Siehr, D. J. (1978). Mechanism of the ninhydrin reaction. Biochemical Education, 6(1), 4–5.
- BYJU’S. (n.d.). Ninhydrin test. https://byjus.com/chemistry/ninhydrin-test/
- Çakar, E., Çakar, S., Toibazarova, A., Syzdykbayev, M., Sydykova, G., Appazov, N., & Özacar, M. (2025). Dye sensitized solar cells applications of Ruhemann’s purple metal complexes. Chemical Methodologies, 9(12), 1143–1153. https://doi.org/10.48309/chemm.2025.535388.1990
- CleanControlling. (2025, July 28). What is “cleaning validation” for medical devices? https://www.cleancontrolling.com/en/news/newsdetails/what-is-cleaning-validation-for-medical-devices
- Comprehensive analytical and forensic evaluation of the ninhydrin reaction: Principles, chemical mechanisms, and multi-disciplinary applications in bioanalytical chemistry. (n.d.).
- Cowan, T. M. (2011). Commentary. Clinical Chemistry, 57(4), 548–549. https://doi.org/10.1373/clinchem.2010.160333
- De Bruijn, A. C. P., Orzechowski, T. J. H., & Wassenaar, C. (2001). Validation of the ninhydrin swab test to monitor cleaning of medical instruments.
- Díaz, J. (2019, October 25). Medical devices: Hygiene monitoring through protein detection. Cleanroom Technology. https://cleanroomtechnology.com/medical-devices-hygiene-monitoring-through-protein-detection–159360
- Drochioiu, G., Sandu, I., Olteanu, G. I., & Mangalagiu, I. (n.d.). Ninhydrin-based forensic investigations I. Fingerprints. International Journal of Criminal Investigation, 1(1), 37–58.
- Filo. (2025, October 31). Give me curtains reaction of alpha amino acids. https://askfilo.com/user-question-answers-smart-solutions/give-me-curtains-reaction-of-alpha-amino-acids-3431333832373535
- Flinn Scientific. (n.d.). Amino acid fingerprints. https://www.flinnsci.com/api/library/Download/a71f79d2e9434d53b0aeb9863e2928d4
- Friedman, M. (2004). Applications of the ninhydrin reaction for analysis of amino acids, peptides, and proteins to agricultural and biomedical sciences. Journal of Agricultural and Food Chemistry, 52(3), 385–406. https://doi.org/10.1021/jf030490p
- Fujifilm Wako. (n.d.). Ninhydrin color development method. https://labchem-wako.fujifilm.com/us/category/00434.html
- Konno, H. (2020). Stain protocol for the detection of N-terminal amino groups during solid-phase peptide synthesis. SYNFORM. https://www.thieme.de/en/thieme-chemistry/synform-detection-of-n-terminal-amino-groups-during-solid-phase-peptide-synthesis-159552.htm
- Lipscomb, I. P., Pinchin, H. E., Collin, R., & Hampden-Smith, K. (2006). The sensitivity of approved Ninhydrin and Biuret tests in the assessment of protein contamination on surgical steel as an aid to prevent iatrogenic prion transmission. Journal of Hospital Infection, 64(3), 288–292. https://doi.org/10.1016/j.jhin.2006.07.007
- Mayo Clinic. (2022, May 13). Phenylketonuria (PKU). https://www.mayoclinic.org/diseases-conditions/phenylketonuria/diagnosis-treatment/drc-20376308
- MedlinePlus. (2022). Phenylketonuria (PKU) screening. https://medlineplus.gov/lab-tests/phenylketonuria-pku-screening/
- Moat, S. J., Schulenburg-Brand, D., Lemonde, H., Bonham, J. R., Weykamp, C. W., Mei, J. V., Shortland, G. S., & Carling, R. S. (2020). Performance of laboratory tests used to measure blood phenylalanine for the monitoring of patients with phenylketonuria. Journal of Inherited Metabolic Disease, 43(2), 179–188. https://doi.org/10.1002/jimd.12163
- Mustansyriah University. (n.d.). Color test for proteins and amino acids. https://www.uomustansiriyah.edu.iq/media/lectures/2/2_2023_01_09!09_16_35_PM.pdf
- NeuLog. (n.d.). Experiment C-28 Beer-Lambert law Ver 3.0.5. https://neulog.com/wp-content/uploads/2014/10/Experiment-C-28-Beer%E2%80%93Lambert-law-Ver-3.0.5.pdf
- Nikoui, K. (2024, February 16). Unveiling hidden clues: The science of ninhydrin fingerprint development. Nikoui & Associates. https://www.nikouiandassociates.com/unveiling-hidden-clues-the-science-of-ninhydrin-fingerprint-development/
- NJ Labs. (2025). Amino acids testing services: Assay, purity, and impurities. https://njlabs.com/our-services/amino-acids-testing/
- Patil, V. P., Devdhe, S. J., Angadi, S. S., Kale, S. H., Phalke, S. D., Shelke, S. D., & Patil, R. H. (2014). Validated spectrophotometric method for the estimation of ketorolac tromithamine in bulk and tablets using ninhydrin: A modified approach. Asian Journal of Research in Chemistry, 7(1), 19–24.
- Powers Scientific, Inc. (2015, September 16). Fingerprint detection with ninhydrin. https://powersscientific.com/fingerprint-detection-with-ninhydrin/
- SATHEE. (n.d.). Chemistry ninhydrin test. https://sathee.iitk.ac.in/article/chemistry/chemistry-ninhydrin-test/
- Schwarz, L., Nat, P., & Frerichs, I. (2002). Advanced solvent-free application of ninhydrin for detection of latent fingerprints on thermal paper and other surfaces. Journal of Forensic Sciences, 47(6), 1274–1277. https://ojp.gov/ncjrs/virtual-library/abstracts/advanced-solvent-free-application-ninhydrin-detection-latent
- Shimadzu. (n.d.). How do you use the Beer-Lambert Law to perform quantitative analysis? https://www.ssi.shimadzu.com/service-support/faq/uv-vis/light-and-theory/10/index.html
- Sigma-Aldrich. (2018). 60017 Kaiser test kit. https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/351/155/60017dat.pdf
- Stauß, A. C., Fuchs, C., Jansen, P., Repert, S., Alcock, K., Ludewig, S., & Rozhon, W. (2024). The ninhydrin reaction revisited: Optimisation and application for quantification of free amino acids. Molecules, 29(14), 3262. https://doi.org/10.3390/molecules29143262
- Testbook. (n.d.). Ninhydrin test: Learn its principle, procedure, & applications. https://testbook.com/chemistry/ninhydrin-test
- UCLA Chemistry. (n.d.). Beer’s law tutorial.
- Uematsu, M., Miyamoto, Y., Shimizu, M., Kajiura, T., Saito, A., Takashina, M., … & Yamamoto, E. (2024). Design and validation of a method for evaluating medical device cleanliness by recovering and quantifying residual proteins on stainless plates. Scientific Reports, 14(1), 21982. https://doi.org/10.1038/s41598-024-72473-1
- Vedantu. (n.d.). Ninhydrin test: Principle, reaction & application. https://www.vedantu.com/chemistry/ninhydrin-test
- Verma, D., Pillai, V. N. R., & Tailor, G. (2020). Role of capping in peptide synthesis. International Journal of Research in Pharmaceutical Sciences, 11(4), 5225–5228. https://doi.org/10.26452/ijrps.v11i4.3134
- Webneel.com. (n.d.). Ninhydrin test: A versatile method for detecting amino acids and proteins. https://webneel.com/sites/default/files/images/blog/fckfile/49326657042.pdf
- Wikipedia. (n.d.). Ninhydrin. https://en.wikipedia.org/wiki/Ninhydrin
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.