What is Neutralization Test?
- The neutralization test is a process used to determine the ability of antibodies to block or reduce the infectivity of a pathogen. When an individual becomes infected with a pathogen, their immune system produces antibodies that recognize and bind to specific parts of the pathogen known as epitopes. Among these antibodies, a subset has the ability to neutralize the pathogen’s infectivity through a process called neutralization.
- Neutralization can occur either in vitro or in vivo. In vitro neutralization tests involve using laboratory animals or tissue culture cells as “indicator systems.” These indicator systems are exposed to the toxin or virus being tested, and the effects of the pathogen on the indicator system are observed. If the effects of the pathogen are neutralized by the presence of specific antibodies, it indicates the presence of neutralizing antibodies in the test sample.
- In the case of toxins, an antiserum containing antibodies that can neutralize the toxin is called an antitoxin. Neutralization tests involving toxins typically measure the reduction of the toxin’s effects on the indicator system when the antitoxin is present.
- Neutralization is a critical aspect of the immune response against pathogens. It refers to the reduction of the harmful effects caused by a particular component, such as viral antigens, viruses, or toxins produced by disease-causing microbes like bacterial exotoxins. Neutralizing antibodies play a crucial role in this process.
- Not all antibodies produced in the body can effectively block the effects of antigens. Only specific antibodies known as neutralizing antibodies have the ability to prevent the uptake of viral particles or antigens by cells and neutralize their activity. These neutralizing antibodies can effectively bind to the pathogen and interfere with its ability to infect host cells or cause harm.
- The neutralization test belongs to both in vitro and in vivo types of antigen-antibody reactions. In vitro tests are conducted in a controlled laboratory environment using isolated components, while in vivo tests involve the use of living organisms, such as animals, to evaluate the neutralizing effects of antibodies.
- Overall, the neutralization test is a valuable tool in immunology and virology research, providing insights into the effectiveness of antibodies in blocking the infectivity of pathogens and understanding the immune response to various diseases.
Principle of Neutralization
The neutralization principle is based on the understanding that certain antibodies or antitoxins can reduce or neutralize the various biological effects caused by enzymes, toxins, and viruses.
There are two main types of neutralization tests:
- Virus Neutralization Test: This type of test is specifically designed for the detection and neutralization of viral activity. Viruses can be cultivated through methods such as egg inoculation, animal inoculation, or cell culture. In the virus neutralization test, specific antibodies against a particular virus are introduced into these growth mediums. The presence of these antibodies halts the growth and replication of the virus. The antibodies target the antigenic determinants present on the surface of the virus, effectively neutralizing its effects.
- Toxin Neutralization Test: Microbes can produce toxins that are capable of causing harm by disrupting various biological functions when injected into the body. However, antitoxins are prepared specifically to counteract the biological effects of these toxins. The toxin neutralization test relies on the utilization of these antitoxins to neutralize the specific toxins. By introducing the antitoxins into the test system, the harmful effects of the toxins can be reduced or nullified.
In both types of neutralization tests, the goal is to assess the ability of specific antibodies or antitoxins to neutralize the effects of the target pathogens. By measuring the reduction or elimination of the biological activities caused by enzymes, toxins, or viruses, researchers can gain valuable insights into the effectiveness of the neutralizing agents and the overall immune response.
The neutralization principle provides a foundation for conducting these tests and contributes to our understanding of how antibodies and antitoxins can counteract the harmful effects of pathogens. By harnessing the power of neutralization, scientists can develop strategies for diagnosing, preventing, and treating diseases caused by various pathogens.
A. Virus Neutralization Test
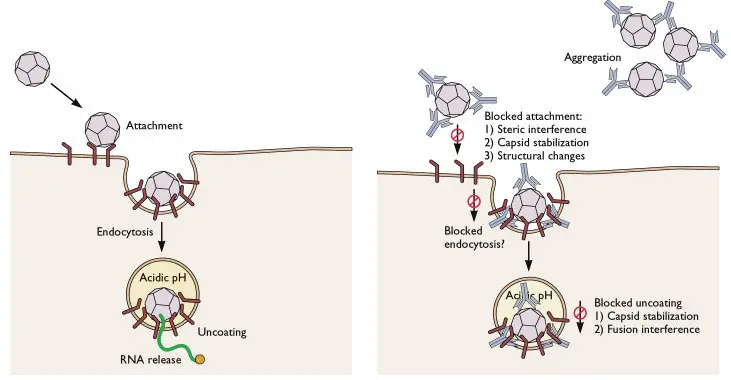
The Virus Neutralization Test is a specific type of neutralization test utilized for virus detection and assessing the neutralization of the biological activities of viruses.
Viruses can be cultivated through various methods such as egg inoculation, animal inoculation, or cell culture. In the Virus Neutralization Test, specific antibodies that are specific to the target virus are introduced into these growth mediums. By doing so, the antibodies have the ability to halt the growth and replication of the viruses. This is the fundamental principle underlying the virus neutralization test.
Viruses possess specific antigenic determinants on their surface, which are recognized by the corresponding antibodies. When these antibodies encounter the virus in the test system, they initiate the process of neutralization. The neutralizing antibodies bind to the antigenic determinants on the virus, preventing its interaction with host cells and thereby neutralizing its effects. This inhibits the virus from causing further damage or infecting cells.
The Virus Neutralization Test is an essential tool for studying the immune response to viral infections, evaluating the efficacy of vaccines, and identifying the presence of specific viruses in diagnostic settings. By measuring the reduction or inhibition of viral activity in the presence of neutralizing antibodies, researchers can assess the effectiveness of the immune response and the potential for viral neutralization.
Two examples of the Virus Neutralization Test include:
- Neutralization of Cytopathic Effect
- Haemagglutination Inhibition Test
1. Neutralization of Cytopathic Effect
- Neutralization of cytopathic effect (CPE) refers to the process of inhibiting or preventing the damage and death of host cells caused by viral infection. Cytopathic effect is the term used to describe the structural changes that occur in host cells when they are invaded by a virus. These changes can range from alterations in cell morphology to cell death.
- When a virus infects host cells, it replicates within the cells and utilizes the cellular machinery for its own replication. This process often leads to the disruption of normal cellular functions and the induction of cytopathic effects. The specific effects can vary depending on the virus and the host cell type. For example, poliovirus is known to induce cytopathic effects in infected cells.
- Neutralization of cytopathic effect involves the use of specific antibodies or antiviral agents to counteract the damaging effects of the virus on the host cells. These neutralizing agents can bind to the virus particles, preventing their attachment to host cells or blocking their entry into cells. By doing so, the neutralizing agents interfere with the viral replication cycle and mitigate the cytopathic effects.
- In the context of neutralization tests, the ability of antibodies or antiviral agents to prevent or reduce cytopathic effects is evaluated. This can be assessed through various methods, such as observing the morphology and viability of infected cells or measuring the reduction in cell death caused by viral infection.
- The neutralization of cytopathic effect is an important aspect of studying viral infections and developing antiviral therapies. By neutralizing the damaging effects of the virus on host cells, it is possible to reduce the severity of the infection, limit the spread of the virus, and improve the overall outcome for the infected individual.
- In summary, neutralization of cytopathic effect refers to the prevention or reduction of the structural changes and cell death caused by viral infection. It involves the use of specific antibodies or antiviral agents to counteract the damaging effects of the virus on host cells, ultimately mitigating the cytopathic effects and improving the outcome of the infection.
Requirements for Neutralization of Cytopathic Effect
The neutralization of cytopathic effect (CPE) requires specific components and conditions to effectively assess the ability of antibodies or antiviral agents to counteract the damaging effects of a virus on host cells. The following are the key requirements for conducting a neutralization assay to evaluate CPE:
- Serum Sample: A serum sample is necessary for the neutralization assay. This serum may be obtained from an individual who has been previously exposed to the virus or has received vaccination against the virus. The serum contains antibodies that can potentially neutralize the virus and prevent or reduce the cytopathic effects.
- Known Viral Suspension: A known viral suspension is required for the assay. This suspension contains a standardized amount of the virus being tested. It serves as the source of the virus to infect the host cells and induce cytopathic effects.
- Cell Culture Suitable for the Virus: A suitable cell culture system is essential for the propagation of the virus and the assessment of cytopathic effects. Different viruses have specific host cell requirements, and the appropriate cell line must be used to support viral replication and exhibit cytopathic effects upon infection.
- Diluting Solution: A diluting solution is used to prepare serial dilutions of the serum sample or other test substances. This allows for the assessment of varying concentrations of neutralizing agents. The diluting solution is typically a buffered medium that provides the necessary conditions for maintaining cell viability and supporting viral replication.
Neutralization of Cytopathic Effect assay Procedure
The neutralization of cytopathic effect (CPE) assay is a procedure used to assess the ability of a serum sample or test substance to neutralize the cytopathic effects induced by a specific virus. The general steps involved in conducting the assay are as follows:
- Serum Collection and Dilution: A serum sample is collected from the patient or individual being tested. The serum is diluted to various concentrations using a diluting solution. This allows for the assessment of different concentrations of neutralizing antibodies present in the serum.
- Mixing with Known Viral Suspension: The diluted serum sample is mixed with a known viral suspension. The viral suspension contains a standardized amount of the target virus. The serum and viral suspension are mixed in equal volumes and thoroughly combined.
- Incubation: The mixture of the diluted serum and viral suspension is then incubated for a specific period of time. The incubation conditions are set to promote viral replication and allow for the interaction between the neutralizing antibodies in the serum and the virus.
- Inoculation in Cell Culture: After the incubation period, the mixture is inoculated onto a suitable cell culture. The cell culture system used should be compatible with the virus being tested. The inoculated cells are typically grown in a culture dish or plate.
- Incubation and Observation: The inoculated cells are incubated under appropriate conditions, which support viral replication and the development of cytopathic effects. The cells are observed periodically for a defined period of time, usually several days, to assess the presence or absence of cytopathic effects. These effects can include changes in cell morphology, detachment of cells from the culture dish, or cell death.
- Control Preparation: In addition to the test samples, a control sample is prepared. The control may include a known positive control, which contains the virus without any neutralizing agent, and a negative control, which may contain a mock or non-specific serum sample.
Result Interpretation
The interpretation of the results from a neutralization assay for cytopathic effects is relatively straightforward. The presence or absence of cytopathic effects after the assay provides information about the neutralizing capacity of the test sample, typically a serum containing antibodies, against the virus being tested.
- Positive Result: If the test sample contains neutralizing antibodies in sufficient concentrations, it will neutralize the viral components present in the assay. As a result, cytopathic effects, such as changes in cell morphology, detachment of cells, or cell death, won’t be observed in the cell culture. This outcome is considered a positive result, indicating that the test sample has neutralizing activity against the virus.
- Negative Result: Conversely, if the test sample does not contain neutralizing antibodies or the concentration of neutralizing antibodies is not sufficient, the viral components will not be effectively neutralized. In this case, the virus will continue to replicate within the cells, leading to the occurrence of cytopathic effects. The observation of cytopathic effects in the cell culture indicates a negative result, suggesting that the test sample lacks significant neutralizing activity against the virus.
It’s important to note that the interpretation of the results should take into account appropriate control samples. These controls may include a positive control, which contains the virus without any neutralizing agent, and a negative control, which may contain a mock or non-specific serum sample. These controls help validate the assay and provide a reference for result interpretation.
In summary, a positive result in the neutralization assay for cytopathic effects indicates that the test sample contains neutralizing antibodies capable of neutralizing the virus, leading to the absence of cytopathic effects. A negative result suggests the absence or insufficient concentration of neutralizing antibodies, leading to the presence of cytopathic effects in the cell culture. Result interpretation is typically based on the comparison with appropriate control samples.
2. Haemagglutination Inhibition Test
- The Haemagglutination Inhibition (HI) test is a serological assay used to measure the presence and quantity of specific antibodies against a virus in a serum sample. It is based on the principle of inhibiting the process of haemagglutination, which refers to the aggregation or clumping of red blood cells by a virus.
- In the haemagglutination process, a virus can bind to red blood cells, causing them to stick together and form visible clumps or aggregates. This phenomenon occurs due to the interaction between viral surface proteins, such as haemagglutinin, and receptors on the surface of red blood cells. The level of haemagglutination can be quantified and serves as an indicator of the presence of the virus or specific antibodies against the virus.
- The Haemagglutination Inhibition test capitalizes on the ability of specific antibodies to prevent or inhibit the virus-induced haemagglutination. In this test, the serum sample is first treated to remove non-specific inhibitors. Then, the serum is serially diluted to create a range of concentrations. Each diluted serum sample is mixed with a standardized amount of the virus that possesses haemagglutination properties.
- If the serum contains specific antibodies against the virus, these antibodies will bind to the viral surface proteins, preventing the virus from attaching to the red blood cells and inhibiting the formation of haemagglutination. As a result, no visible clumping or aggregation of red blood cells will occur.
- The endpoint of the Haemagglutination Inhibition test is determined by observing the highest dilution of the serum that completely inhibits haemagglutination. This dilution represents the quantity of specific antibodies in the serum sample.
- The Haemagglutination Inhibition test is widely used in virology and immunology to assess immunity to viral infections, particularly for viruses that exhibit haemagglutination properties. It provides a convenient and reliable method for quantifying the levels of specific antibodies against a virus in a serum sample.
- In summary, the Haemagglutination Inhibition test measures the presence and quantity of specific antibodies against a virus by assessing their ability to inhibit the process of haemagglutination. By preventing the clumping or aggregation of red blood cells induced by the virus, the test provides valuable information about the level of immunity to a specific viral infection.
Requirements for Haemagglutination Inhibition Test
The Haemagglutination Inhibition (HI) test requires specific components and preparations to successfully assess the presence and quantity of neutralizing antibodies against a virus. The following are the key requirements for conducting a Haemagglutination Inhibition test:
- Serum Sample: A serum sample is obtained from the patient or individual being tested. The serum contains antibodies, including neutralizing antibodies, that may be present due to a previous viral infection or vaccination. The serum is the primary component used to assess the inhibitory effect on haemagglutination.
- Known Virus Suspension: A standardized and well-characterized suspension of the virus being tested is required. The virus used should be capable of inducing haemagglutination, which refers to the clumping of red blood cells. This known virus suspension serves as the target for the neutralizing antibodies present in the serum sample.
- Red Blood Cell (RBC) Suspension: A suspension of red blood cells is necessary for the haemagglutination process. The source of the RBCs depends on the specific virus being tested. For example, if testing for the Rubella virus, RBCs from newborn chickens may be used as they are susceptible to agglutination by the Rubella virus. The choice of RBC source should be appropriate for the virus being tested.
- Diluting Solution: A diluting solution, such as saline or a buffered solution, is used to prepare serial dilutions of the serum sample. The dilutions are necessary to assess the inhibitory effect of the antibodies present in the serum on the haemagglutination process. The diluting solution should be compatible with the virus and maintain the viability of the virus and RBCs during the test.
Procedure of Haemagglutination Inhibition Test
The Haemagglutination Inhibition (HI) test follows a general procedure to assess the presence and quantity of neutralizing antibodies in a serum sample. The steps involved in conducting a Haemagglutination Inhibition test are as follows:
- Serum Sample Collection: A serum sample is collected from the suspected patient or individual. The serum contains antibodies, including neutralizing antibodies, which will be tested for their inhibitory effect on haemagglutination.
- Serial Dilution: The serum sample is serially diluted in saline solutions. Typically, a range of dilutions is prepared to cover a broad spectrum of antibody concentrations in the sample. Serial dilutions help determine the titer or quantity of neutralizing antibodies in the serum.
- Addition of Virus: A standardized amount of the virus being tested is added to each dilution of the serum sample. The virus used should possess haemagglutination properties, meaning it can cause clumping of red blood cells.
- Incubation: The mixture of the diluted serum sample and virus is incubated under appropriate conditions. This allows the antibodies present in the serum to interact with the virus.
- Addition of RBC Suspension: After the incubation period, a suspension of red blood cells (RBCs) from a specific source is added to the mixture. The RBCs serve as the target for the haemagglutination process induced by the virus.
- Mixing and Observation: The components, including the serum-virus mixture and RBC suspension, are thoroughly mixed. The mixture is then observed for the presence or absence of haemagglutination. Haemagglutination refers to the clumping or aggregation of the red blood cells induced by the virus.
If the serum sample contains neutralizing antibodies in sufficient concentrations, these antibodies will bind to the viral surface proteins and prevent the virus from attaching to the red blood cells. As a result, no visible clumping or aggregation of the RBCs will occur, indicating inhibition of haemagglutination.
The endpoint of the Haemagglutination Inhibition test is determined by identifying the highest dilution of the serum that completely inhibits haemagglutination. This highest dilution represents the titer or concentration of neutralizing antibodies in the serum sample.
In summary, the procedure for the Haemagglutination Inhibition test involves serial dilution of the serum sample, addition of the virus, incubation, addition of RBC suspension, thorough mixing, and observation of haemagglutination. The presence or absence of haemagglutination indicates the inhibitory effect of neutralizing antibodies present in the serum sample.
Result Interpretation of Haemagglutination Inhibition Test
The interpretation of results in the Haemagglutination Inhibition (HI) test is based on the presence or absence of haemagglutination, which indicates the inhibitory effect of neutralizing antibodies present in the patient’s serum. The following guidelines can be used to interpret the results:
- Positive Test: If haemagglutination is not observed in the serum sample, it is considered a positive test. This result suggests that the patient’s serum contains neutralizing antibodies specific to the virus being tested. The neutralizing antibodies bind to different binding sites on the virus, preventing its attachment to the red blood cells and inhibiting haemagglutination.
- Negative Test: If haemagglutination is observed in the serum sample, it is considered a negative test. This result suggests the absence or insufficient levels of neutralizing antibodies in the serum. Without neutralizing antibodies, the virus can bind to the red blood cells and induce haemagglutination.
- Determination of Severity or Antibody Amount: The extent of haemagglutination observed at different dilutions of the serum sample can provide additional information. If haemagglutination is observed at higher dilutions, it suggests a lower concentration of neutralizing antibodies in the serum, indicating a less severe infection or a lower amount of neutralizing antibodies. Conversely, if haemagglutination is inhibited even at higher dilutions, it suggests a higher concentration of neutralizing antibodies, indicating a more robust immune response or a more severe infection.
The Haemagglutination Inhibition test is useful for assessing the presence and quantity of neutralizing antibodies in the patient’s serum. The absence of haemagglutination indicates the presence of neutralizing antibodies and a positive test result. The extent of haemagglutination at different dilutions can provide insights into the severity of the infection or the concentration of neutralizing antibodies in the serum.
Applications of Virus Neutralization Test
The Virus Neutralization Test has several applications in the field of virology and diagnostics. Some of the key applications are as follows:
- Detection of Viral Infections: The Virus Neutralization Test is widely used to detect and diagnose viral infections. By testing the presence of neutralizing antibodies in a patient’s serum, it can determine if the individual has been exposed to a specific virus, such as measles, mumps, influenza, or the novel coronavirus causing COVID-19. This test helps in confirming the infection and assessing its severity.
- Assessment of Immune Response: The Virus Neutralization Test is valuable in evaluating the immune response of an individual against a viral infection. It can determine the presence and quantity of neutralizing antibodies in the serum, which provide vital information about the immune system’s ability to counteract the virus. This assessment can help in monitoring the progression of the infection, evaluating the effectiveness of treatments or vaccines, and predicting immunity against future exposures.
- Vaccine Development: The Virus Neutralization Test plays a crucial role in the development and evaluation of vaccines against viral diseases. By measuring the neutralizing antibodies generated in response to a vaccine, researchers can determine its effectiveness in preventing viral infection. This test helps in assessing the vaccine’s ability to induce a robust immune response and produce neutralizing antibodies, which are essential for neutralizing the virus and preventing disease.
- Seroprevalence Studies: Seroprevalence studies aim to determine the prevalence of a specific virus within a population. The Virus Neutralization Test is a valuable tool for such studies as it can detect the presence of neutralizing antibodies in a large number of samples. These studies help in understanding the extent of viral exposure within a population, identifying areas with higher infection rates, and informing public health strategies.
- Research and Surveillance: The Virus Neutralization Test is used in research settings to study viral pathogens, their behavior, and their interaction with the host immune system. It provides insights into the mechanisms of viral neutralization and helps in identifying potential targets for antiviral therapies. Additionally, the test aids in surveillance efforts by monitoring the prevalence and spread of viral infections in specific populations or regions.
In summary, the Virus Neutralization Test has broad applications in diagnosing viral infections, assessing immune response, vaccine development, seroprevalence studies, and virology research. It plays a critical role in understanding viral diseases, monitoring their spread, and informing public health interventions.
B. Toxin Neutralization Test
The Toxin Neutralization Test is a laboratory assay designed to evaluate the effectiveness of antitoxins in neutralizing the harmful effects of toxins produced by microbial pathogens. Toxins are substances released by microbes that can cause significant damage to various biological functions within the body when injected or exposed to them. However, antitoxins are specific antibodies produced to counteract and reduce the biological effects of these toxins.
The principle of the Toxin Neutralization Test is based on utilizing these antitoxins to neutralize the toxic effects of the target toxin.
Requirements
The requirements for conducting the Toxin Neutralization Test include:
- Egg Yolk Agar: Egg Yolk Agar is a specific type of agar medium used for this test. It contains nutrients and egg yolk, which provides a source of lecithin. Lecithin is a component found in egg yolks that can be dissolved by certain bacterial toxins, such as alpha-toxin produced by bacteria like Clostridium perfringens. The presence of egg yolk in the agar allows for the detection of lecithinase activity.
- Known Antitoxin: A known antitoxin is required for the test. This antitoxin is a specific antibody preparation produced against the toxin of interest. It should be well-characterized and have a known neutralizing activity against the toxin being tested. The known antitoxin serves as a positive control and is used to assess the neutralizing capacity of the test sample.
- Inoculum of Test Organism: The test organism, which produces the toxin of interest, is required for the assay. This organism can be a bacterial strain or other microorganisms known to produce the specific toxin. The inoculum of the test organism is prepared and added to the test medium, such as the Egg Yolk Agar, for the toxin production.
- Anaerobic Jar or Incubator: The Toxin Neutralization Test is typically performed under anaerobic conditions, as some toxin-producing bacteria thrive in oxygen-deprived environments. Therefore, an anaerobic jar or an incubator equipped with the necessary conditions for maintaining anaerobic conditions, such as low oxygen and high carbon dioxide levels, may be required during incubation of the test samples.
Procedure of Nagler’s reaction
Nagler’s reaction is a laboratory test used to detect the presence of lecithinase activity produced by certain bacteria, particularly Clostridium perfringens. Lecithinase is an enzyme that can dissolve lecithin, a component found in egg yolk. This test is named after its developer, George Henry Falkiner Nuttall and Arthur Edward Boycott Nagler.
The procedure for Nagler’s reaction is as follows:
- Preparation of Egg Yolk Agar Plate: An agar plate containing egg yolk is prepared. The egg yolk provides a source of lecithin for the test.
- Dividing the Plate: A line is drawn on the agar plate to divide it into two halves.
- Application of Antitoxin: Antitoxin, a known specific antibody against the lecithinase toxin, is spread on one-half of the agar plate. This half serves as the test area.
- Streaking of Test Organism: The test organism, which is known to produce lecithinase, is streaked on the entire agar plate, including both the antitoxin-treated half and the untreated half. The streaking is performed in a way that allows the growth of the organism on the plate.
- Incubation: The agar plate is then placed in an anaerobic incubator at a temperature of 37 °C (98.6 °F) for one to two days. The anaerobic environment is necessary for the growth and activity of lecithinase-producing bacteria, such as Clostridium perfringens.
Result Interpretation
The interpretation of results in Nagler’s reaction is based on the presence or absence of an opaque zone on the agar plate. The following guidelines can be used to interpret the test results:
- Positive Result: If an opaque zone is observed in the area of the agar plate without antitoxin treatment (antitoxin-free area), and no opaque zone is observed in the area where antitoxin was applied (antitoxin inoculated area), the test is considered positive. This indicates the presence of lecithinase activity produced by the test organism.
- Negative Result: Conversely, if an opaque zone is not observed in either the antitoxin-free area or the antitoxin inoculated area, the test is considered negative. This suggests the absence of lecithinase activity produced by the test organism.
It is important to note that the activity of the alpha-toxin (lecithinase) is neutralized by the antitoxin, resulting in no opaque zone in the antitoxin inoculated area. However, in the antitoxin-free area, the toxin activity is not neutralized, allowing the lecithinase to degrade the lecithin present in the egg yolk agar and form an opaque zone.
The presence or absence of the opaque zone in the respective areas of the agar plate serves as an indicator of lecithinase activity. The presence of an opaque zone in the antitoxin-free area indicates a positive result, suggesting the presence of lecithinase-producing bacteria. Conversely, the absence of an opaque zone in both areas indicates a negative result, indicating the absence of lecithinase activity.
Proper interpretation of the results in Nagler’s reaction is crucial for identifying organisms that produce lecithinase, such as Clostridium perfringens.
Applications of Toxin Neutralization Test
The toxin neutralization test finds applications in both in vivo and in vitro settings. Here are some of its common uses:
In vivo use:
- Schick test: The Schick test is frequently employed to demonstrate immunity to diphtheria infection. It involves injecting a small amount of diphtheria toxin into the skin and observing the reaction. If the individual is immune to diphtheria, no reaction occurs.
- Neutralization of toxins produced by Clostridium welchii: The toxin neutralization test can be used to neutralize the toxins produced by Clostridium welchii, a bacterium known for causing gas gangrene. By adding antitoxins specific to the toxins produced by Clostridium welchii, the harmful effects of the toxins can be neutralized.
In vitro use:
- Rapid detection of Clostridium spp by Nagler’s reaction: Nagler’s reaction, a form of toxin neutralization test, is used for the rapid detection of Clostridium species. By observing the presence or absence of an opaque zone in the agar plate after incubation, the presence of lecithinase enzyme-producing Clostridium spp can be determined. This method provides a quick and efficient way to identify these organisms.
- Anti Streptolysin O (ASO) test: The ASO test is based on toxin neutralization and is used to detect antibodies against the Streptolysin O toxin produced by Streptococcus pyogenes. By measuring the levels of anti-Streptolysin O antibodies in a patient’s serum, the test can aid in diagnosing a recent or past streptococcal infection.
In summary, the toxin neutralization test has various applications in both in vivo and in vitro settings. It is used for demonstrating immunity to diphtheria, neutralizing toxins produced by specific bacteria such as Clostridium welchii, rapidly detecting Clostridium species, and measuring antibodies against toxins like Streptolysin O. These applications contribute to the diagnosis, monitoring, and understanding of diseases caused by toxin-producing pathogens.
Applications of Neutralization Test
The neutralization test has several applications in the field of immunology and microbiology. Here are some of its key applications:
- Determination of pathogenic components: The neutralization test is utilized to determine the presence and activity of pathogenic components such as toxins and viruses. By introducing antibodies or antitoxins specific to these components, the neutralizing ability can be assessed, aiding in the identification and differentiation of their pathogenicity.
- Study of antigenic relations: The test is valuable in studying the antigenic relations between different viruses and toxins. By examining the ability of antibodies to neutralize different strains or variants, researchers can gain insights into the antigenic similarities and differences among these pathogens.
- Assessment of vaccine efficacy: The neutralization test is used to evaluate the effectiveness of vaccines in producing immunity. By measuring the ability of vaccine-induced antibodies to neutralize the targeted pathogens, researchers can determine the vaccine’s ability to confer protection against infection.
- Evaluation of drug effectiveness: In addition to vaccines, the neutralization test can be employed to assess the effectiveness of drugs or therapeutic agents. By testing their ability to neutralize the activity of pathogens or toxins, researchers can evaluate the potential of these drugs to inhibit infection or mitigate the harmful effects of toxins.
- Determination of antitoxin potency: The neutralization test plays a vital role in determining the potency of antitoxins. By measuring the ability of antitoxins to neutralize the toxic effects of specific microbial toxins, researchers can assess the strength and efficacy of the antitoxin preparations.
Advantages of Neutralization Test
The neutralization test offers several advantages in the field of immunology and microbiology. Here are some of its key advantages:
- Higher sensitivity: The neutralization test has a higher sensitivity compared to some other diagnostic methods. It can detect low levels of neutralizing antibodies or antigens, making it a valuable tool for detecting infections or assessing immune responses.
- Higher specificity: The test also exhibits higher specificity, meaning it can accurately identify the presence of specific antibodies or antigens. This specificity helps in distinguishing between different pathogens or strains, providing precise diagnostic information.
- Detection of various viral strains: In virus neutralization tests, the neutralizing antibodies used can detect the target virus along with its various strains. This is particularly beneficial in situations where viruses can undergo antigenic variation or mutate, ensuring that the test can still accurately detect and identify the virus.
- Quantitative assessment: The neutralization test allows for quantitative assessment of neutralizing antibodies or antigens. It provides information about the level of neutralizing activity, which can be useful for evaluating the strength of immune responses, monitoring the effectiveness of vaccines, or determining the potency of antitoxins.
- Diagnostic and research versatility: The neutralization test is applicable to a wide range of pathogens, including viruses and bacteria that produce toxins. This versatility makes it a valuable tool in both diagnostic settings and research laboratories, enabling the detection, characterization, and evaluation of various pathogens and their components.
Limitations of Neutralization Test
While the neutralization test is a valuable tool in immunology and microbiology, it also has certain limitations that should be considered. Here are some of the key limitations of the neutralization test:
- Time delay in antibody generation: Neutralizing antibodies take time to develop in the body after an infection or immunization. This means that the test may not be suitable for early diagnosis of acute infections, as it may take days or weeks for detectable levels of neutralizing antibodies to be produced.
- Need for skilled personnel: Performing neutralization tests requires trained and skilled individuals who have expertise in handling and manipulating viruses, toxins, and other test components. The complexity of the test and the need for precise techniques make it important to have personnel with the necessary expertise to ensure accurate and reliable results.
- Labor-intensive process: The neutralization test requires a significant amount of work, including the preparation of specific test materials, such as viral suspensions, antitoxins, and cell cultures. The test involves multiple steps and careful execution, which can be time-consuming and resource-intensive.
- Challenges in production and management of materials: Some of the materials required for the neutralization test, such as specific viral strains, antitoxins, and cell cultures, can be challenging to produce and manage. The availability and quality of these materials can vary, which may impact the reliability and reproducibility of the test results.
- Requirement for high-level biosafety measures: Working with live viruses and toxins in the neutralization test necessitates highly protective laboratory conditions and adherence to strict biosafety protocols. The handling of infectious agents can pose risks to laboratory personnel, requiring proper training, containment facilities, and adherence to safety guidelines to minimize the potential hazards.
FAQ
What is a neutralization test?
A neutralization test is a laboratory method used to determine the ability of specific antibodies to neutralize the effects of pathogens, such as viruses or toxins.
What are the applications of a neutralization test?
Neutralization tests have various applications, including diagnosing viral infections, assessing immune responses, evaluating vaccine effectiveness, studying antigenic relations, and testing the ability of antitoxins to neutralize toxins.
What are the advantages of a neutralization test?
Some advantages of a neutralization test include its high sensitivity and specificity, the ability to detect different viral strains, quantitative assessment of neutralizing activity, and versatility in diagnostic and research settings.
How long does it take to generate neutralizing antibodies?
The generation of neutralizing antibodies in the body after an infection or immunization can vary. It typically takes several days to weeks for detectable levels of neutralizing antibodies to develop.
How does a neutralization test work?
In a neutralization test, the pathogen or its components are mixed with the test sample containing antibodies. If the antibodies can neutralize the pathogen, it will prevent its biological activity or binding to target cells.
Who performs a neutralization test?
Neutralization tests are typically performed by trained and skilled laboratory professionals who have expertise in handling and manipulating pathogens, performing the required techniques, and interpreting the results accurately.
What materials are needed for a neutralization test?
The specific materials required for a neutralization test may vary depending on the pathogen being tested. Generally, it includes serum samples, known viral suspensions or toxins, appropriate cell cultures or indicator systems, and diluting solutions.
Is a neutralization test time-consuming?
Performing a neutralization test can be time-consuming as it involves multiple steps, such as sample preparation, mixing with pathogens, incubation, and result interpretation. The complexity of the test can contribute to the overall time required.
Are there any safety precautions associated with a neutralization test?
Yes, working with live viruses or toxins in a neutralization test requires adherence to strict biosafety measures and the use of appropriate containment facilities. Protective equipment and proper handling procedures are essential to minimize the risk to laboratory personnel.
Can a neutralization test be used for any type of pathogen?
Neutralization tests are primarily used for viruses and toxins produced by microbes. They may not be applicable to all pathogens, and the suitability of a neutralization test depends on the specific characteristics of the pathogen being studied.
References
- Thullier P., Sesardic D.T. (2010) Neutralization Tests. In: Kontermann R., Dübel S. (eds) Antibody Engineering. Springer Protocols Handbooks. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-01144-3_45.
- Melissa Ann Bourgeois, J. Lindsay Oaks, Equine Infectious Diseases (Second Edition), 2014.
- Hitchner S. B. (1973). A virus neutralization screening test: its limitations in classifying field isolates of infectious bronchitis virus. Avian pathology: journal of the W.V.P.A, 2(2), 103–109. https://doi.org/10.1080/03079457309353788
- Parija S.C., (2009), Textbook of Microbiology and Immunology, 2nd edition, Elsevier, a division of Reed Elsevier India Private Limited.
- https://jamanetwork.com/journals/jama/article-abstract/295465
- https://experiments.springernature.com/articles/10.1007/978-3-642-01144-3_45
- https://www.rockland.com/resources/neutralization-assay-protocol/