What is Neisseria meningitidis?
- Neisseria meningitidis, commonly known as the meningococcus, is a Gram-negative bacterium that plays a crucial role in causing serious infections such as meningitis and meningococcemia. It is classified as a coccus due to its spherical shape and typically appears as a diplococcus, forming pairs of these round cells.
- This bacterium is an exclusively human pathogen, which means it cannot infect animals, making it a significant cause of disease in humans. While about 10% of adults carry the bacterium in their nasopharynx without showing symptoms, it can lead to severe diseases in certain conditions. In fact, Neisseria meningitidis is responsible for the only form of bacterial meningitis that occurs in epidemic outbreaks, particularly in regions such as sub-Saharan Africa and parts of Asia. Although the disease can occur anywhere, it is most common in areas where people live in close quarters, such as in military barracks, dormitories, and overcrowded urban settings.
- The bacterium is primarily transmitted through respiratory droplets and saliva, which can spread through activities like coughing, sneezing, kissing, or sharing contaminated items, such as drinking glasses or chewing on toys. Additionally, sexual transmission has been documented, with cases of urethritis occurring in men due to oral sex.
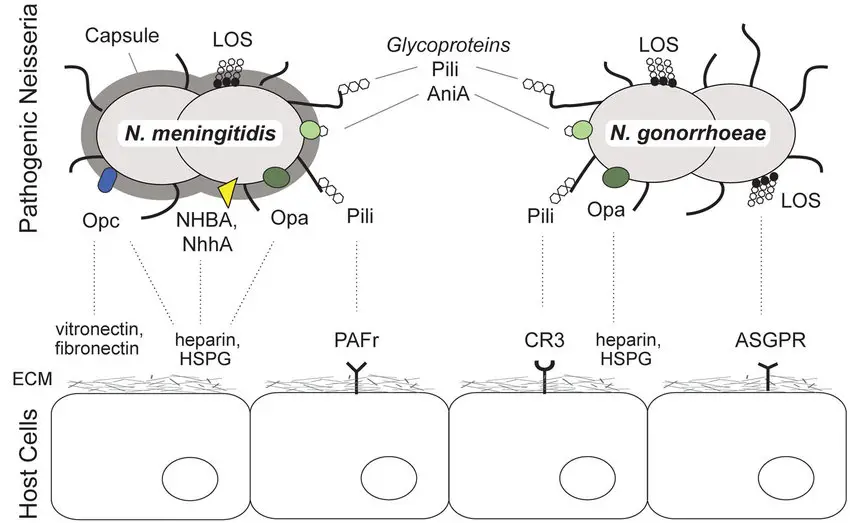
- Once inside the body, N. meningitidis uses specialized appendages called pili to adhere to host cells, allowing the bacterium to colonize the upper respiratory tract. Pili are thin, hair-like structures that help the pathogen stick to mucosal surfaces. Additionally, surface-exposed proteins like Opa and Opc contribute to the bacterium’s ability to interact with host cells. These virulence factors play a critical role in the bacterium’s capacity to invade and cause infection.
- In terms of disease, N. meningitidis can cause meningitis, an infection of the membranes surrounding the brain and spinal cord, and meningococcemia, a severe bloodstream infection that can result in organ failure and shock. Despite the high rates of asymptomatic carriage, when the bacterium causes disease, the consequences can be severe, with developmental impairment or death occurring in approximately 10% of infected individuals. This highlights the importance of vaccination and early intervention in preventing these life-threatening conditions.
Geographical Distribution of Neisseria meningitidis
Neisseria meningitidis, the bacteria responsible for meningococcal disease, is a global threat. However, its impact varies across different regions, with certain serogroups causing more significant outbreaks and epidemics than others. The distribution of this pathogen is not uniform, and the occurrence of cases is often influenced by factors such as geography, socioeconomic conditions, and age groups.
- Serogroup A is most commonly associated with large-scale epidemics, particularly in sub-Saharan Africa. Central African countries, in particular, experience alarming attack rates of 400-500 cases per 100,000 people. These epidemics often strike in cycles, causing devastating outbreaks in these regions.
- Serogroup B causes both epidemics and localized outbreaks. It has been responsible for meningococcal diseases in various parts of the world, especially in developed countries.
- Serogroup C is linked to localized outbreaks, and though it can cause epidemics, these are less frequent compared to serogroups A and B.
- Serogroups B, C, and Y dominate in developed countries, accounting for 90% of meningococcal disease cases in these areas. These regions see fewer large-scale epidemics but still experience ongoing outbreaks.
The global distribution of Neisseria meningitidis is complex. While developing countries face higher risks for epidemic outbreaks, developed countries contend with more sporadic cases primarily caused by serogroups B and C. The disease is more prevalent in children under 5 years and elderly individuals, making age an important factor in disease occurrence.
Habitat of Neisseria meningitidis
Neisseria meningitidis thrives as a human pathogen, primarily residing in the upper respiratory tract.
- Nasopharynx and oral cavity are the main habitats where N. meningitidis is found.
- People can carry the bacteria asymptomatically, meaning they don’t show signs of infection but can still spread the bacteria to others.
- Asymptomatic carriage rates can range from 1% to 40% in different populations.
- The highest carriage rates are seen in school-age children, young adults, and those with lower socioeconomic status.
Reservoir, Source, and Transmission of Neisseria meningitidis Infection
Neisseria meningitidis is a human-exclusive pathogen. There are no animal reservoirs for this bacteria.
- Humans are the only known reservoir for meningococcal infection.
- The primary source of infection is nasopharyngeal secretions. This includes droplets from the nose and throat.
- Airborne transmission occurs when droplets from an infected person are inhaled by someone nearby.
Crowded living conditions increase the risk of transmission.
- Family members living in close quarters or those in military barracks and prisons are particularly vulnerable.
- Older adults are also at a higher risk.
Morphology of Neisseria meningitidis
Neisseria meningitidis, the causative agent of bacterial meningitis and meningococcemia, has distinct morphological features that are essential for identification and understanding of its pathogenicity.
- Shape:
N. meningitidis appears as Gram-negative cocci, typically spherical or slightly oval in shape. These bacteria are often seen in pairs, with the adjacent sides of the cocci flattened, a characteristic that gives them a distinct “diplococcus” appearance. - Size:
The bacteria measure between 0.6–0.8 µm in diameter, making them relatively small in size compared to some other bacterial pathogens. - Intracellular Location:
In samples from infected individuals, particularly pus cells, the bacteria are often found intracellular within polymorphonuclear leukocytes (PMNs). This suggests the bacteria are engulfed by immune cells during infection. - Capsule:
Capsule presence is notable in freshly isolated strains of N. meningitidis. The capsule plays a crucial role in the bacterium’s ability to evade the host’s immune response, contributing to its virulence. - Nonmotility and Nonsporing:
N. meningitidis is nonmotile and does not form spores. This distinguishes it from some other bacteria that utilize motility or spore formation as part of their survival strategy in harsh environments.
Culture and Biochemical Reactions of Neisseria meningitidis
Neisseria meningitidis, the bacterium responsible for meningitis, requires specific conditions for optimal growth. Below is a detailed description of its growth characteristics and biochemical behavior:
Culture Characteristics
- Oxygen Requirements:
N. meningitidis is a strict aerobe, meaning it requires oxygen for growth. It thrives best in an environment with 5% CO2, and temperatures between 36°C and 39°C. The optimal pH for its growth is between 7.4 and 7.6. - Growth Media:
This bacterium is fastidious, meaning it has complex nutritional needs. It does not grow well on standard media. Instead, it flourishes on enriched media like blood agar, chocolate agar, and Mueller–Hinton agar. Blood or serum in these media helps neutralize inhibitory substances, allowing the bacterium to grow effectively. It is important to note that these media don’t provide additional nutrients but create a favorable environment for the bacteria. - Blood Agar:
On blood agar, N. meningitidis forms small, round colonies, about 1–2 mm in diameter. These colonies appear gray and translucent with smooth edges at 24 hours of incubation. After 48 hours, the colonies increase in size and develop a raised center with a transparent margin, but they do not exhibit hemolysis (i.e., no breakdown of red blood cells). Strains with a large polysaccharide capsule produce mucoid colonies. - Selective Media:
For isolation from clinical samples containing mixed bacterial flora, selective media like Thayer-Martin medium and New York City medium are commonly used. These media contain antibiotics such as vancomycin, colistin, nystatin, and trimethoprim, which help inhibit the growth of other bacteria, promoting the growth of N. meningitidis.
Biochemical Reactions
- Oxidase and Catalase Positive:
N. meningitidis is oxidase positive and catalase positive, which are essential tests for its preliminary identification. These tests help differentiate N. meningitidis from other oxidase-positive bacteria, such as Alcaligenes spp., Aeromonas spp., Vibrio spp., Campylobacter spp., and Pseudomonas spp.- Oxidase Test:
The oxidase test can be performed in two ways:- A 1% oxidase reagent solution (tetramethyl paraphenylene-diamine-dihydrochloride) is applied to the bacterial culture. If the bacteria are oxidase positive, the colonies will turn deep purple.
- Alternatively, a few colonies are rubbed on a filter paper strip moistened with the oxidase reagent. A deep purple color will develop almost immediately if the bacteria are oxidase positive.
- Oxidase Test:
- Fermentation Patterns:
N. meningitidis ferments glucose and maltose, producing acid but no gas. It does not ferment sucrose or lactose, which helps in distinguishing it from other species. These fermentation patterns are crucial for the final identification of Neisseria species. - Other Biochemical Reactions:
The bacterium does not produce hydrogen sulfide and does not reduce nitrates, further aiding in its identification and differentiation from other organisms.
Cell Wall Components and Antigenic Structure of Neisseria meningitidis
The structure of Neisseria meningitidis, including its cell wall and antigenic features, plays a critical role in its pathogenicity and ability to cause disease. Here’s a breakdown of these key components:
Cell Wall Components
- Endotoxin (LPS):
The cell wall of N. meningitidis contains a lipopolysaccharide (LPS), also known as endotoxin. This LPS is chemically identical to that found in enteric bacilli, which are commonly associated with intestinal infections. The presence of endotoxin in the cell wall contributes to the bacterium’s ability to cause inflammation and is a key factor in its virulence.
Antigenic Structure
- Serogroups:
N. meningitidis is categorized into 13 distinct serogroups based on group-specific capsular polysaccharide antigens. These serogroups are labeled A, B, C, D, X, Y, Z, W135, 29E, H, I, K, and L. The serogroups play a significant role in the pathogenicity and epidemiology of the disease.- Serogroups A, B, and C: These are the primary culprits behind meningitis epidemics and outbreaks worldwide.
- Serogroups Y and W135: These are more commonly associated with disease outbreaks than serogroups X and Z.
- Non-pathogenic Strains: Meningococci that lack these group-specific antigens are generally considered nonpathogenic, meaning they do not typically cause disease.
- Serotypes:
Each serogroup of N. meningitidis can be further divided into serotypes. These are identified based on differences in the proteins present on the outer membrane and the oligosaccharide part of lipooligosaccharide (LOS). For instance:- Group A: Contains a single serotype.
- Groups B and C: These groups consist of multiple serotypes, making them more diverse in their antigenic profiles.
- Role of Serotyping: The identification of specific serotypes is valuable in pinpointing virulent strains for epidemiological studies. This allows for better tracking of outbreaks and understanding the spread of the disease.
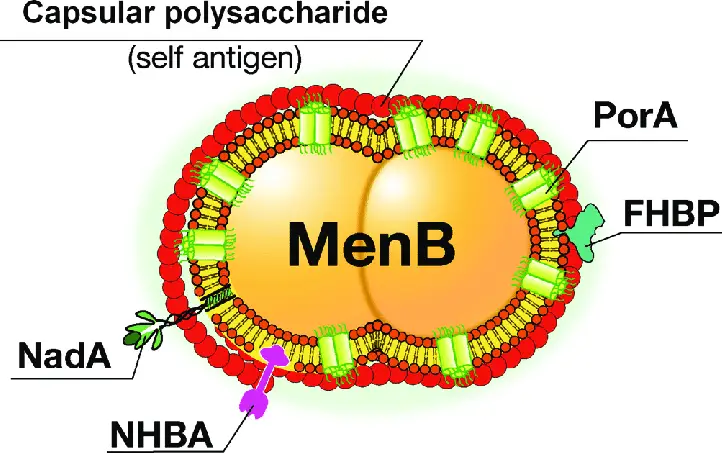
Virulence Factors of Neisseria meningitidis
Neisseria meningitidis relies on several key virulence factors to cause infection and evade the immune system. These factors are critical for the bacterium’s survival and pathogenicity in the human host.
Key Virulence Factors
- Capsular Polysaccharide:
- N. meningitidis is encased in a polysaccharide capsule, which acts as a major defense mechanism.
- The capsule is antiphagocytic, meaning it helps the bacterium avoid being engulfed and destroyed by immune cells like leukocytes.
- This protection allows the meningococci to survive within the phagocytic vesicle of immune cells, where they can multiply and later migrate to subepithelial spaces.
- The presence of the capsule is a critical factor in the bacterium’s ability to cause disease.
- LOS Endotoxin:
- Found in the outer membrane, LOS endotoxin plays a major role in the pathogenesis of meningococcal infections.
- This endotoxin is responsible for causing blood vessel damage, which is a hallmark of the infection.
- The endotoxin consists of two antigenic determinant components: a protein and a carbohydrate. Both contribute to the virulence.
- Continuous production and release of endotoxin by N. meningitidis contribute to the severe endotoxin reaction often seen in meningococcal disease, causing systemic symptoms like shock and vascular damage.
- IgA Protease:
- IgA protease is another enzyme produced by N. meningitidis that enhances its ability to infect the host.
- The protease cleaves secretory IgA, an antibody found in mucosal areas like the upper respiratory tract.
- By degrading IgA, the protease helps the bacteria evade immune defense and adhere to the epithelial cells in the upper respiratory system, enabling further colonization.
Each of these factors—capsular polysaccharide, LOS endotoxin, and IgA protease—work in tandem to allow N. meningitidis to evade immune responses, colonize its host, and cause severe invasive diseases like meningitis.
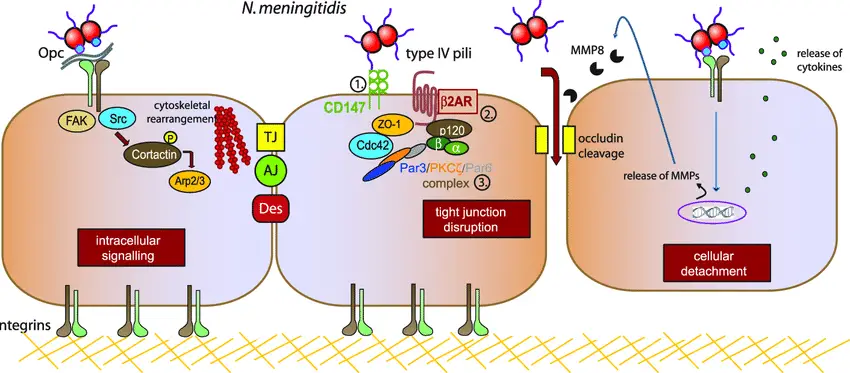
Pathogenesis of Neisseria meningitidis
The journey of Neisseria meningitidis begins with colonization of the nasopharynx, which is often asymptomatic. However, when conditions are right, the bacteria can break through host defenses and invade deeper tissues, leading to severe systemic infection, including meningitis.
Steps in the Pathogenesis Process
- Colonization of the Nasopharynx:
- Meningococci first settle in the nasopharynx, where they can persist without causing noticeable symptoms.
- The nasopharyngeal infection is typically subclinical, meaning it doesn’t always trigger a noticeable immune response in the host.
- Invasion of the Bloodstream:
- Once inside the bloodstream, the meningococci face the challenge of evading immune defenses like antibodies, complement, and neutrophils.
- These bacteria can escape immune surveillance and travel to distant parts of the body, including the central nervous system (CNS).
- The precise mechanism by which meningococci reach the subarachnoid space remains unclear, but their ability to evade the host’s immune system is crucial in this process.
- Direct Spread to the CNS:
- Meningococci can also spread directly from the nasopharynx to the CNS without the need for the bloodstream.
- Once they reach the CNS, they multiply and thrive, largely unchecked by the host’s immune system. Immunoglobulins, neutrophils, and complement seem to have limited effect on controlling bacterial growth within the cerebrospinal fluid (CSF).
- The uncontrolled multiplication of bacteria in the CSF causes a cascade of meningeal inflammation, leading to severe meningitis.
- Clinical Disease Development:
- While most individuals experience a brief asymptomatic nasopharyngeal carriage, in some cases, the bacteria invade the circulation, resulting in clinical disease.
- This progression typically begins with a localized infection that can evolve into a much more severe systemic infection, such as meningococcal sepsis or meningitis.
Clinical Syndromes of Neisseria meningitidis
Meningitis, meningococcemia, and other syndromes are the primary clinical manifestations caused by Neisseria meningitidis. These infections can range from mild to life-threatening, with varying degrees of severity depending on the presence of complications and the timeliness of treatment.
Meningitis
- Meningococcal meningitis typically affects children and young adults.
- It starts as a febrile illness with symptoms like headache, stiff neck, and fever.
- Common signs also include lethargy or drowsiness, but confusion, delirium, and stupor can appear in rarer cases.
- Severe cases may progress to mental obtundation, stupor, or even coma due to increased intracranial pressure.
- Prognosis is generally good when treated promptly with appropriate antimicrobial therapy, with patients often making a full recovery.
- Comatose patients or those with local neurological findings tend to have a worse prognosis.
Meningococcemia
- Meningococcemia, with or without meningitis, is life-threatening and presents acutely with fever and a petechial rash.
- The rash starts as small spots on the trunk and lower extremities, often coalescing into large hemorrhagic lesions.
- One of the most severe forms of meningococcemia is Waterhouse-Friderichsen syndrome, characterized by:
- Severe disseminated intravascular coagulation (DIC)
- Shock
- Multisystem organ failure, including adrenal gland destruction
- This syndrome is almost always fatal and is often linked to C5-C9 complement deficiencies.
- The vascular damage in Waterhouse-Friderichsen syndrome is primarily due to the action of LOS endotoxin released by the bacteria.
Other Syndromes
- Around 10% of patients with meningococcal disease develop nonsuppurative arthritis, commonly affecting the knee joint. This is believed to be immunologically mediated and appears within the first 48 hours of treatment.
- Recurrent meningococcal meningitis is seen in individuals with hereditary deficiencies in various complement system components.
- Additional conditions linked to meningococcal infection include:
- Meningococcal pneumonia (likely from aspiration of the bacteria)
- Septic arthritis
- Purulent pericarditis
- Endophthalmitis
Laboratory Diagnosis of Neisseria meningitidis Infection
The lab diagnosis of Neisseria meningitidis infection relies heavily on identifying the bacteria in clinical specimens through microscopy and culture techniques.
- Specimens of choice include cerebrospinal fluid (CSF) and blood for diagnosing meningitis.
- Nasopharyngeal swabs are used to detect carriers of the bacteria.
- CSF is collected through lumbar puncture, while blood is obtained by venipuncture under strict aseptic conditions.
- It’s crucial not to refrigerate CSF, as the cold temperature can kill other bacteria, like Haemophilus influenzae, that may be present in the sample.
- Transport of CSF and blood should be quick to prevent any degradation. CSF should be sent immediately to the lab, while blood is placed in glucose broth or sodium taurocholate broth for incubation.
- Nasopharyngeal specimens are collected using sterile swabs and transported in Stuart’s transport medium.
Cerebrospinal Fluid (CSF)
CSF analysis reveals several typical changes in cases of meningococcal meningitis:
- The CSF becomes more turbid compared to normal.
- The white blood cell count increases to over 1000 WBCs per liter, with PMN cells being predominant.
- Total protein levels rise, while glucose levels drop, a condition known as hypoglycorrhachia (lower than 60% of normal blood glucose levels).
- Intracranial pressure may also increase.
CSF Processing Steps
- The first part of CSF is centrifuged. The smear from the deposit is Gram stained. The supernatant is tested for meningococcal antigens.
- The second part is used for direct culture of the bacteria.
- The third part is incubated with glucose broth overnight, then subcultured onto blood agar and chocolate agar.
Microscopy
- Gram staining of CSF is a quick and effective way to detect N. meningitidis.
- Meningococci appear as Gram-negative diplococci, primarily inside leukocytes, though some may be seen extracellularly.
- Gram staining can identify N. meningitidis in approximately 50% of patients with meningococcal meningitis.
- In cases of fulminant meningococcemia, Gram stains of peripheral blood can reveal Gram-negative diplococci in the buffy coat.
Culture
- Culture confirms the diagnosis by isolating N. meningitidis from CSF, blood, and other specimens.
- CSF is inoculated on nonselective media like blood agar or chocolate agar, then incubated at 35–36°C with 5% CO2 for 18–24 hours. The resulting colonies will be small, round, translucent, and convex with a smooth surface.
- Blood cultures are done by inoculating glucose broth or sodium taurocholate broth and incubating at 35–36°C. Subcultures are made on blood or chocolate agar. Daily subcultures are performed for 4–7 days. Blood culture is often positive in the early stage of meningitis or in cases of meningococcemia.
Other Specimens
- Nasopharyngeal swabs and petechial exudates are processed similarly to CSF samples.
Identification of Bacteria
- N. meningitidis can be identified based on several characteristics and morphological features.
- Serogrouping is done through slide agglutination using specific hyperimmune serum.
Antigen Detection
- Detection of soluble polysaccharide antigen in the CSF can be used to diagnose meningococcal meningitis.
- Common tests include counter-current immunoelectrophoresis, latex agglutination, and bacterial coagglutination tests.
- These tests are particularly helpful when bacterial counts in the CSF are low. However, antigen detection is less effective in detecting Group B meningococci, as these strains are nonimmunogenic and don’t react with specific antibodies.
Serodiagnosis
- Indirect hemagglutination and ELISA tests are used to detect antibodies against the polysaccharide antigen in the serum.
- These tests are helpful for diagnosing chronic meningococcal infections, particularly when cultures come back negative for meningococci.
Treatment of Neisseria meningitidis Infection
The treatment of Neisseria meningitidis infection hinges on the timely use of appropriate antibiotics to prevent complications, including meningococcal meningitis and meningococcemia.
- Penicillin G is the first-line treatment for meningococcal disease.
- The MIC (minimum inhibitory concentration) for penicillin typically ranges from 0.01 to 0.05 µg/mL for meningococcal isolates.
- Intravenous administration is preferred to ensure rapid delivery and effectiveness.
- Other antibiotic options include:
- Chloramphenicol, useful for patients who are allergic to penicillin.
- Rifampicin, erythromycin, and tetracycline can also be considered, though they’re typically used less frequently.
- Cephalosporins like ceftriaxone, cefotaxime, and cefuroxime are effective and commonly used for bacterial meningitis.
- Ceftriaxone has the added benefit of eradicating nasopharyngeal carriage of N. meningitidis, reducing the risk of further spread.
- What to avoid:
- Meningococci are not susceptible to vancomycin or polymyxin.
- Resistance to sulfadiazine (MIC 0.128 µg/mL) has been documented, making it less reliable in some cases.
Prevention and Control of Neisseria meningitidis Infection
Preventing and controlling Neisseria meningitidis infections revolves around both chemoprophylaxis and immunoprophylaxis to stop the spread and protect individuals at risk.
- Chemoprophylaxis:
- The primary goal is to prevent secondary cases of meningococcal disease among close contacts of infected individuals.
- Antibiotics help interrupt person-to-person transmission by eradicating the asymptomatic nasopharyngeal carrier state, reducing the chance of spreading the bacteria.
- Common antibiotics used include:
- Sulfonamides
- Rifampin
- Minocycline
- Ciprofloxacin
- Ceftriaxone
- Ciprofloxacin is not recommended for children due to concerns about cartilage damage observed in animal studies.
- Immunoprophylaxis (Vaccination):
- Vaccination is a critical preventive measure, especially for the capsular polysaccharides of groups A, C, Y, and W135 meningococci.
- These group-specific vaccines are effective in preventing meningococcal disease caused by these particular serogroups.
- Textbook of Microbiology and Immunology – Textbook by Parija SC
- https://en.wikipedia.org/wiki/Neisseria_meningitidis
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.