Mucic acid test is a specific chemical test used for the identification of galactose and galactose containing sugars like lactose. It is also referred to as galactaric acid test. It is based on the oxidation reaction of sugars with concentrated nitric acid. In this test the sugar sample is heated with concentrated nitric acid which acts as a strong oxidizing agent and converts the terminal aldehyde and primary alcohol groups into carboxylic acid groups.
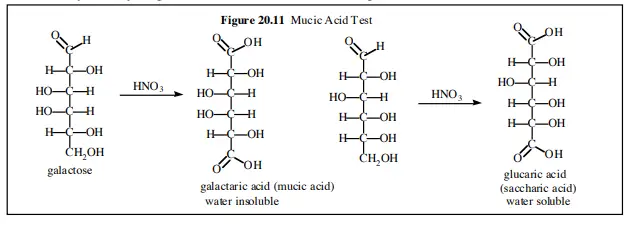
During this process galactose is oxidized to form mucic acid (meso-galactaric acid). It is the process where the formed mucic acid shows internal molecular symmetry and this is referred to as meso compound. Due to this symmetry the mucic acid molecules are packed closely and becomes insoluble in water. Other sugars like glucose on oxidation form glucaric acid which remains soluble in water and does not produce precipitate.
The positive result of mucic acid test is indicated by the formation of white crystalline precipitate at the bottom of the test tube after heating and cooling. These crystals are rod shaped and are insoluble in water. Thus the test is mainly used to differentiate galactose and lactose from other monosaccharides and disaccharides.
Principle of Mucic acid test
The principle of mucic acid test is based on the oxidation of galactose and galactose containing carbohydrates by concentrated nitric acid. In this test concentrated nitric acid acts as a strong oxidizing agent and on heating it oxidizes both the aldehyde group at carbon 1 and the primary alcohol group at carbon 6 of the sugar molecule. Due to this oxidation the sugar is converted into a dicarboxylic acid which is also known as aldaric acid.
In case of D-galactose the oxidation results in the formation of galactaric acid which is commonly called mucic acid. It is the process where the specific stereochemical arrangement of galactose leads to the formation of a meso compound having internal molecular symmetry. Due to this symmetry the mucic acid molecules are closely packed and becomes insoluble in water and acidic medium and hence precipitates out as white crystalline solid.
Other sugars like glucose on oxidation form dicarboxylic acids such as glucaric acid which are asymmetric in nature and remain soluble in water and therefore do not form precipitate. Lactose also gives positive mucic acid test because during heating with nitric acid the glycosidic bond is hydrolysed releasing galactose which is then oxidized to mucic acid.
Objective of Mucic acid test
- To detect the presence of D-galactose in the given carbohydrate sample.
- To identify galactose containing sugars such as lactose which on hydrolysis releases galactose.
- To differentiate galactose from other monosaccharides like glucose and mannose based on formation of insoluble mucic acid.
- To help in identification of galactose containing compounds present in food samples and laboratory preparations.
- To use as a supportive test in screening of galactose metabolism related disorders like galactosemia.
Requirements for Mucic acid test
- Concentrated nitric acid (HNO₃) – it acts as the oxidizing agent in the test.
- Test sample – carbohydrate solution (usually 1%) containing galactose or lactose.
- Distilled water – used for preparation of sugar solution and dilution.
- Test tubes – clean and dry test tubes are required for carrying out reaction.
- Test tube stand – used for holding the test tubes during heating and cooling.
- Pipette or dropper – for measuring and transferring acid and sugar solution.
- Water bath or flame – used for heating the reaction mixture carefully.
- Glass rod – used for scratching the inner surface of test tube to initiate crystal formation if required.
- Safety equipments – laboratory gloves, goggles and lab coat are used as nitric acid is corrosive.
Procedure of Mucic acid test
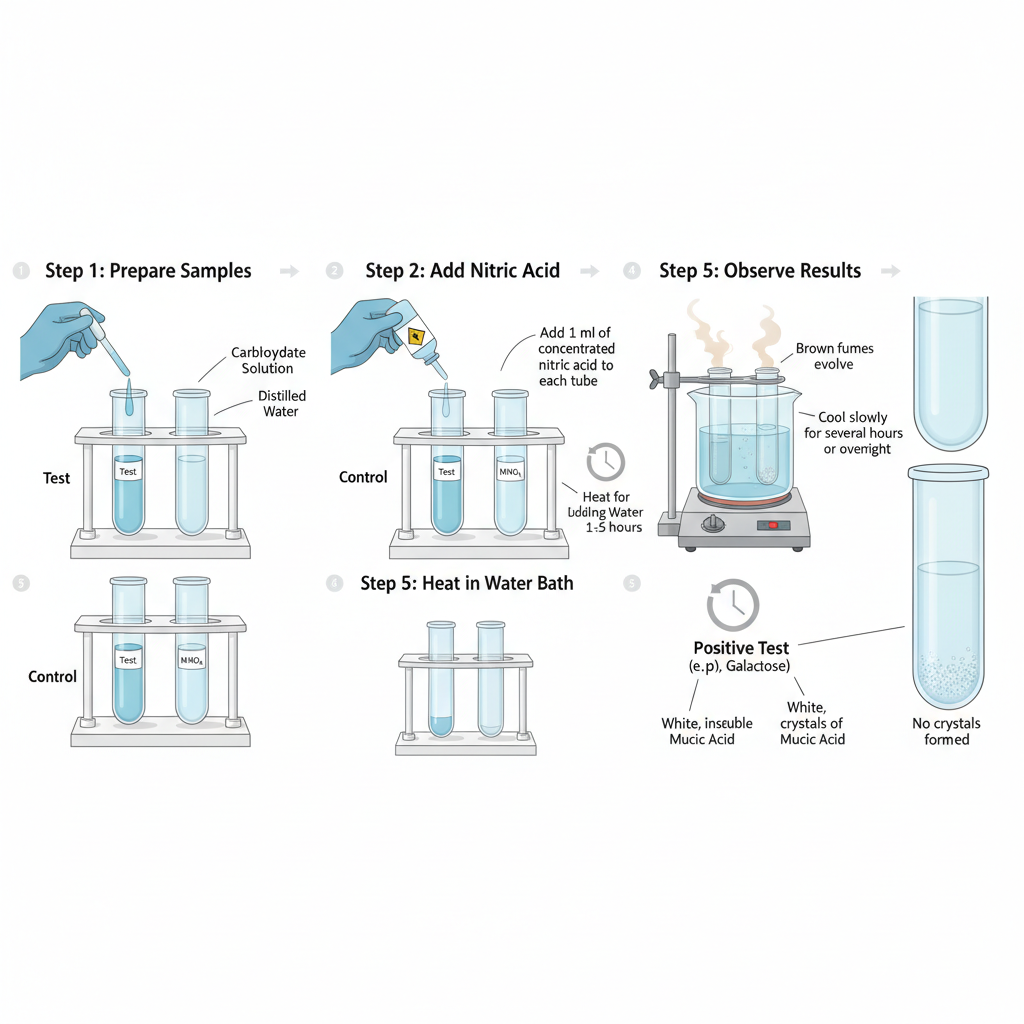
- Take clean and dry test tubes and label them properly for test sample and control.
- Add about 5 ml of the carbohydrate solution to the test tube. Distilled water is taken in another test tube as control.
- Add 1 ml of concentrated nitric acid carefully to each test tube. Mix the contents gently by swirling.
- Place the test tubes in a boiling water bath and heat for about 1 to 1½ hours. Heating is continued till the volume of the solution is reduced to nearly one third.
- During heating brown fumes may be evolved and care should be taken while performing the step.
- After heating remove the test tubes and allow them to cool slowly to room temperature.
- Keep the test tubes undisturbed for several hours or overnight for crystallization.
- Observe the bottom of the test tube for formation of white crystalline precipitate indicating positive mucic acid test.
Results of Mucic acid test

- Positive result (+)
- White insoluble precipitate is formed at the bottom of the test tube.
- On microscopic observation the crystals appear rod shaped in nature.
- It indicates the presence of D-galactose or galactose containing sugars like lactose.
- The positive result is due to formation of mucic acid which is insoluble in water.
- Negative result (–)
- No precipitate is formed and the solution remains clear.
- It indicates absence of galactose and galactose containing compounds.
- Sugars like glucose and mannose give negative result as their oxidation products remain soluble.
- Limitation of the test
- The test does not differentiate between free galactose and galactose obtained from lactose.
Uses of Mucic acid test
- It is used to detect the presence of D-galactose in a given carbohydrate sample.
- It is used for identification of galactose containing sugars such as lactose.
- It helps in differentiation of galactose and lactose from other sugars like glucose, fructose and mannose.
- It is used in clinical biochemistry as a supportive test for screening of galactosemia.
- It is applied in food analysis to check the presence of galactose or lactose in food samples.
- It is also used in laboratory and industrial preparations for identification of galactose containing compounds.
Limitations of Mucic acid test
- It does not differentiate between free galactose and galactose present in disaccharides like lactose.
- Galactose released from complex carbohydrates also gives positive result making differentiation difficult.
- Impurities present in the sample may give false positive result.
- The test requires use of concentrated nitric acid which is corrosive and hazardous.
- Toxic brown fumes are released during heating which needs proper safety precautions.
- The test is time consuming as crystallization requires several hours or overnight standing.

FAQ
1. What is the mucic acid test?
Mucic acid test is a specific chemical test used for the identification of galactose and galactose containing sugars. It is also known as galactaric acid test. In this test the carbohydrate is oxidized by concentrated nitric acid and formation of insoluble mucic acid crystals indicates positive result.
2. What is the principle of the mucic acid test?
The principle of mucic acid test is based on oxidation of galactose by concentrated nitric acid. Nitric acid oxidizes the aldehyde group and primary alcohol group of galactose to form a dicarboxylic acid called mucic acid. Due to internal molecular symmetry mucic acid is insoluble in water and precipitates as white crystals.
3. What is the procedure for the mucic acid test?
In mucic acid test the carbohydrate solution is taken in a test tube and concentrated nitric acid is added to it. The mixture is heated in a boiling water bath till the volume is reduced. After heating the solution is cooled and kept undisturbed for crystallization. Formation of white crystalline precipitate indicates positive test.
4. What does the mucic acid test detect?
The mucic acid test detects the presence of D-galactose and galactose containing carbohydrates in the given sample.
5. What sugars give a positive mucic acid test?
Sugars containing galactose such as galactose itself and lactose give positive mucic acid test. Some other galactose containing compounds may also give positive result.
6. How are the results of the mucic acid test interpreted?
Formation of white insoluble crystalline precipitate at the bottom of the test tube indicates positive result. Absence of precipitate and clear solution indicates negative result.
7. Is the mucic acid test specific for galactose?
The mucic acid test is highly specific for galactose and galactose containing sugars. Other common sugars like glucose and mannose do not form insoluble mucic acid.
8. Why does lactose give a positive mucic acid test?
Lactose gives positive mucic acid test because on heating with concentrated nitric acid it is hydrolysed to glucose and galactose. The released galactose is then oxidized to mucic acid.
9. What are the objectives of the mucic acid test?
The objective of mucic acid test is to detect galactose and galactose containing sugars and to differentiate them from other carbohydrates.
10. What are the uses of the mucic acid test?
The test is used for identification of galactose and lactose. It is used in carbohydrate analysis, food analysis and as a supportive test in screening of galactose metabolism disorders.
11. What are the disadvantages of the mucic acid test?
The test is time consuming and requires concentrated nitric acid which is hazardous. It does not differentiate free galactose from galactose present in lactose.
12. What is galactaric acid?
Galactaric acid is a dicarboxylic acid formed by oxidation of galactose. It is also called mucic acid and is insoluble in water due to its symmetrical structure.
13. What reagents are used in the mucic acid test?
The main reagent used in mucic acid test is concentrated nitric acid. Distilled water and the carbohydrate sample are also required.
14. How is mucic acid formed during the test?
Mucic acid is formed by oxidation of galactose when heated with concentrated nitric acid. The aldehyde and primary alcohol groups are converted into carboxylic acid groups.
15. How can the mucic acid test distinguish between different sugars?
The mucic acid test distinguishes sugars based on solubility of their oxidation products. Galactose forms insoluble mucic acid while other sugars form soluble oxidation products and do not give precipitate.
- Ataman Kimya. (n.d.). Mucic Acid. Retrieved from https://atamankimya.com
- Barth, D., & Wiebe, M. G. (2017). Enhancing fungal production of galactaric acid. Applied Microbiology and Biotechnology, 101(10), 4033–4040. https://doi.org/10.1007/s00253-017-8159-y
- Caton, K. A. (n.d.). Mucic and Barfoeds Test [PowerPoint presentation]. SlideShare.
- Centurion University of Technology and Management. (n.d.). Qualitative Analysis of Carbohydrates [Courseware].
- Environmental Health & Safety, University of Washington. (n.d.). Nitric Acid Safety Focus Sheet. Retrieved from https://www.ehs.washington.edu
- Expert Report. (n.d.). The Mucic Acid Test—Principles of Stereochemical Specificity, Standardized Procedure, and Analytical Interpretation.
- Hamburg, B. (2015, April 27). Nitric Acid Safety Tips & Health Hazards. VelocityEHS.
- Install, J., Zupanc, A., Kim, S., Wang, W., Kemell, M., Huber, G. W., & Repo, T. (2025). Selective oxidation of glucose–galactose syrup to gluconic and galactonic acids. Green Chemistry, 27, 6725–6733. https://doi.org/10.1039/D5GC01381J
- Karothu, D. P., Tahir, I., Majhi, S. M., Ahmed, E., Catalano, L., Hickey, N. T., Weston, J., Guerin, S., & Naumov, P. (2025). The ultrastiff crystals of mucic (galactaric) acid. Chemical Science. https://doi.org/10.1039/d5sc05888k
- LookChem. (n.d.). Cas 526-99-8, Mucic Acid. Retrieved from https://www.lookchem.com
- Pasquale, A., Chiacchio, M. A., Acciaretti, F., & Cipolla, L. (2024). The oxidation of d‐galactose into mucic acid (galactaric acid): experimental and computational insights towards a bio‐based platform chemical. Asian Journal of Organic Chemistry.
- Sapkota, A. (2022, September 5). Mucic Acid Test- Definition, Principle, Procedure, Result, Uses. Microbe Notes.
- Study.com. (n.d.). What is a positive result for mucic acid test?
- ThomasTKtungnung. (n.d.). Mucic acid Test | Galactaric acid Test Practical Experiment [Video]. YouTube.
- Tonguia, T. A. (n.d.). Mucic Acid Test [Document]. Scribd.
- Wikipedia contributors. (n.d.). Mucic acid. Wikipedia, The Free Encyclopedia.