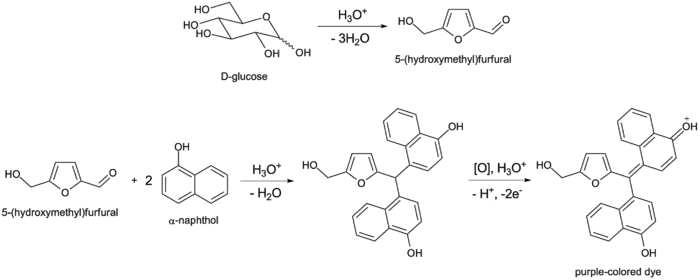
This is a test that is common for all carbohydrate larger than the tetroses. The test works on the basis that pentoses as well as hexoses can be dehydrated using conc. sulfuric acid, resulting in furfural or hydroxyl-methyl furfural or hydroxyl methyl furfural. These compounds condense with a-naphthol to create a purple condensation product.
Molisch’s Test is a type of chemical test that can be used to determine carbohydrate content in a given sample. Molisch’s test is named in honor of the Czecho-Austrianian botanist Hans Molisch, who is recognized as the one who discovered it. Molisch’s test is based on the addition to Molisch’s reagent (a solution of – naphthol and alcohol) in the test analyte. This is followed by the addition of a small amount in concentrated H2SO4 (sulphuric acid) to the mix.
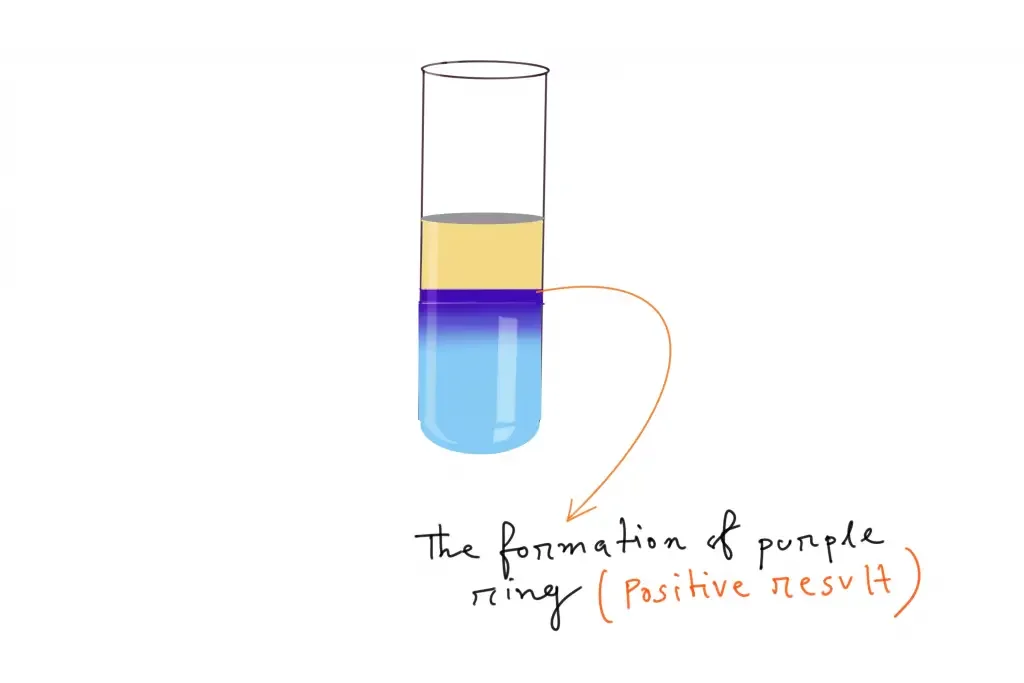
The appearance of a purple or a red-purplish ring at the junction of H2SO4, the analyte, and Molisch’s reagent mix confirms the presence of carbohydrates within the analytical sample.
Objectives of Molisch Test
- To detect the presence of carbohydrates in the given sample.
- To identify monosaccharides disaccharides and polysaccharides by a general group reaction.
- To differentiate carbohydrates from other biomolecules like proteins and lipids.
- To use the test as a preliminary screening test for carbohydrates before specific tests.
- To detect glycosyl containing compounds such as glycoproteins glycolipids and nucleic acids which release sugars on acid hydrolysis.
Molisch’s Test Principle
Molisch test is based on the principle of dehydration of carbohydrates by strong mineral acid. When concentrated sulphuric acid is added to the test solution, the carbohydrates present in the sample is first hydrolysed into simple sugars. These monosaccharides then undergo dehydration by the action of acid, where pentose sugars are converted into furfural and hexose sugars are converted into hydroxymethyl furfural.
These dehydration products then react with Molisch reagent (α-naphthol). In this reaction the aldehyde compounds condense with α-naphthol to form a violet or purple coloured complex. This coloured ring is formed at the junction of acid and aqueous layer. The appearance of violet ring indicates the presence of carbohydrates in the given sample.
Molisch’s Test Reaction
Monosaccharides disaccharides and polysaccharides (except trioses as well as tetroses)will result in positive reactions, and glycoproteins as well as nucleic acids have a positive reaction since all of these compounds are hydrolyzed into monosaccharides with strong mineral acids. Pentoses are then dehydrated to furfural, while hexoses are dehydrated to 5-hydroxymethylfurfural. Any of these aldehydes when present they will be condensed with 2 molecules of a-naphthol and produce a purple-colored compound as shown in the glucose example:
Requirements
Reagents
- Molisch reagent (α-naphthol dissolved in ethanol).
- Concentrated sulphuric acid (H₂SO₄).
- Distilled water.
Apparatus and Glassware
Test Materials and Controls
- Test sample suspected to contain carbohydrate.
- Positive control (known carbohydrate solution such as glucose).
- Negative control (distilled water).
Safety Requirements
- Fume hood for handling concentrated acid.
- Protective goggles or face shield.
- Acid resistant gloves.
- Lab coat and closed footwear.
Procedure of Molisch Test
- Take about 2 ml of the given test solution in a clean and dry test tube.
- Add 2–3 drops of Molisch reagent to the test tube and mix the contents properly.
- The test tube is then kept in a slanting position.
- About 1–2 ml of concentrated sulphuric acid is carefully added along the side of the inclined test tube using a pipette.
- The acid is allowed to settle at the bottom forming a separate layer and the solution is not mixed.
- The junction of the two layers is observed for the formation of a violet or purple coloured ring, which indicates the presence of carbohydrates.
Result Interpretation of Molisch’s Test
- Positive result
- Formation of a violet or purple coloured ring at the junction of the two layers.
- This indicates the presence of carbohydrates in the given sample.
- Monosaccharides disaccharides and polysaccharides give positive result.
- Glycoproteins and nucleic acids may also give positive result due to release of sugars on hydrolysis.
- Negative result
- No violet or purple ring is observed at the junction.
- This indicates absence of carbohydrates in the given sample.
- Triose and tetrose sugars do not respond to this test and give negative result.
- Based on time of appearance of ring
- Immediate appearance of violet ring indicates presence of monosaccharides.
- Delayed appearance of ring indicates presence of disaccharides or polysaccharides.
- Abnormal or false results
- Green coloured ring indicates presence of impurities.
- Black or dark ring indicates charring of sugar due to excess heat or improper addition of acid.


Uses of Molisch Test
- It is used for the general detection of carbohydrates in a given sample.
- It is used to identify monosaccharides disaccharides and polysaccharides.
- It is used to differentiate carbohydrates from proteins and lipids.
- It is used as a preliminary or group test for carbohydrates before specific tests.
- It is used for detection of carbohydrates in food samples and plant extracts.
- It is used to detect glycoproteins glycolipids and nucleic acids due to presence of sugar part.
- It is commonly used for educational and laboratory demonstration purpose.
Limitations of Molisch Test
- It is not specific for carbohydrates and may give positive result with other substances.
- Glycoproteins nucleic acids and some organic acids may also give positive result.
- Trioses and tetroses do not respond to this test and give negative result.
- It cannot differentiate between monosaccharides disaccharides and polysaccharides.
- Improper addition of concentrated acid may cause charring of the sample.
- Impurities in reagents may interfere with the colour development.
- Use of concentrated sulphuric acid makes the test hazardous.
Advantages of Molisch Test
- It is a general group test for detection of carbohydrates.
- It can detect monosaccharides disaccharides and polysaccharides.
- It is a sensitive test and can detect small amount of carbohydrate.
- It can detect sugars present in free form or in combined form.
- The test is simple and easy to perform.
- The result is easily visible by formation of a violet or purple ring.
- It can be used as a rapid preliminary screening test for carbohydrates.
- Aakash Educational Services Limited. (n.d.). Molisch’s Test in Chemistry: Definition, Types and Importance. Retrieved from https://www.aakash.ac.in/important-concepts/chemistry/molischs-test
- BYJU’S. (n.d.). Molisch’s Test Principle. Retrieved from https://byjus.com/chemistry/molischs-test/
- Expert Analysis and Protocol. (n.d.). The Molisch’s Test (A General Assay for Carbohydrate Detection). [Source text provided].
- Faisal, E., Nassr, D., & Farahan, I. (2022). Qualitative analysis of Carbohydrate: Molisch’s Test. AL-Mustaqbal University College, Department of Medical Physics. Retrieved from https://www.uomus.edu.iq/img/lectures21/MUCLecture_2022_1033417.pdf
- Harper College. (n.d.). The Molisch Test – Carbohydrates. Retrieved from https://dept.harpercollege.edu/chemistry/chm/100/dgodambe/thedisk/carbo/molisch/molisch.htm
- Mohanlal Sukhadia University (MLSU). (n.d.). Molisch’s Test. Retrieved from https://mlsu.ac.in/econtents/776_Molisch%E2%80%99s%20Test.pdf
- Professor Drew Collop. (n.d.). Molisch’s Test for Carbohydrates 2.0 [Video]. YouTube. Retrieved from https://www.youtube.com/watch?v=Qkqk8V_iinM
- Sapkota, A. (2022, September 17). Molisch Test- Definition, Principle, Procedure, Result, Uses. Microbe Notes. Retrieved from https://microbenotes.com/molisch-test/
- Testbook. (n.d.). Molischs Test Learn Its Principle, Reagents, Procedure and Observations. Retrieved from https://testbook.com/chemistry/molischs-test
- UC Merced Environmental Health and Safety. (n.d.). Standard Operating Procedure: Sulfuric Acid. Retrieved from https://chemistry.ucmerced.edu/sites/g/files/ufvvjh611/f/page/documents/ejm_sulfuric_acid.pdf
- Unacademy. (n.d.). Notes on Molisch’s test. Retrieved from https://unacademy.com/content/neet-ug/study-material/biology/molischs-test/
- Vedantu. (n.d.). Molisch Test: Principle, Procedure & Results Explained (R. Singla, Rev.). Retrieved from https://www.vedantu.com/chemistry/molisch-test
- Westlab. (2023, September 14). Safety Protocols For Handling Sulfuric Acid in Laboratories. Retrieved from https://www.westlab.com.au/blog/safety-protocols-for-handling-sulfuric-acid-in-laboratories
- Wikipedia. (n.d.). Molisch’s test. Retrieved from https://en.wikipedia.org/wiki/Molisch%27s_test
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.