What is Molecule?
A molecule is a fundamental unit in the realm of chemistry, representing a group of two or more atoms bound together by chemical bonds. These bonds arise due to interactions between the electrons of the participating atoms. Delving deeper into the nature and significance of molecules:
- Basic Composition:
- Atoms: The foundational units of matter, atoms consist of a nucleus (containing protons and neutrons) surrounded by electrons that orbit in specific shells or valence orbits.
- Electron Interactions: Electrons, especially those in the outermost shell, play a pivotal role in bond formation. Their interactions, either through sharing or transferring, lead to the formation of molecules.
- Types of Molecular Bonds:
- Covalent Bonds: Formed when atoms share electrons, covalent bonds can be single, double, or triple, based on the number of shared electrons. Such bonds are integral to biology due to their strength and energy-storing capability. For instance, the energy stored in covalent bonds of food molecules is released upon digestion, facilitated by enzymes and other microorganisms.
- Ionic Bonds: When one atom donates an electron to another, the resultant charged entities (ions) attract each other to form ionic bonds. However, these bonds do not lead to the formation of molecules in the strictest sense.
- Nature of Molecules:
- Homonuclear Molecules: Comprising atoms of a single element, like oxygen in O2.
- Heteronuclear Molecules: Composed of different elements, such as water (H2O) with two hydrogen atoms and one oxygen atom.
- Diatomic Molecules: Molecules with just two atoms, which can be homonuclear like hydrogen (H2) or heteronuclear like water (H2O).
- Significance in Life:
- Carbon-based Molecules: Carbon’s ability to form four covalent bonds makes it central to life, leading to the formation of diverse molecules in conjunction with elements like hydrogen (H2), oxygen (O2), and nitrogen (N2).
- Adenosine Triphosphate (ATP): A quintessential molecule in living beings, ATP stores and releases energy, crucial for various biological processes.
- Historical Perspective:
- The concept of molecules has ancient roots, but rigorous scientific exploration began in the 17th century. Pioneers like Robert Boyle, Amedeo Avogadro, and Linus Pauling have significantly advanced our understanding, leading to the modern fields of molecular physics and chemistry.
In essence, molecules are the building blocks of matter, arising from the intricate dance of atomic electrons. Their diverse structures and interactions underpin the vast array of substances and life processes we observe.
Definition of Molecule
A molecule is a group of two or more atoms bonded together, representing the smallest fundamental unit of a chemical compound that retains the chemical properties of that compound.
Characteristics Of Molecules
Molecules, as fundamental units in the realm of chemistry, exhibit specific attributes that define their nature and behavior. The following elucidates the primary characteristics of molecules:
- Definitive Structural Units: Molecules represent the most rudimentary units of a substance that retain distinct chemical and physical attributes. They serve as the foundational building blocks of matter, encapsulating the essence of the substance they constitute.
- Inherent Size and Mass: Every molecule possesses an intrinsic size and mass, determined by the number and type of atoms it encompasses. This inherent mass is pivotal in various chemical reactions and processes.
- Atomic Composition and Ratio: Molecules are formed when two or more atoms, either of the same element or different elements, coalesce through chemical bonds. The proportion of these constituent atoms within a molecule remains constant for a given compound, ensuring its unique identity.
- Loss of Substance Property upon Disintegration: A molecule’s integrity is crucial for the preservation of the properties of the substance it represents. When a molecule disintegrates, it yields individual atoms. These resultant atoms exhibit chemical and physical traits that diverge significantly from the original molecule.
In essence, molecules are quintessential entities in the chemical domain, characterized by their unique composition, size, and the properties they bestow upon substances. Their stability and integrity are paramount for the preservation of the inherent characteristics of the materials they form.
Types of Biological Molecules
Biological molecules, often referred to as biomolecules, are the fundamental entities that underpin the structure and function of living organisms. These molecules are primarily composed of elements such as carbon, oxygen, nitrogen, and phosphorous. They can range from simple molecules like water (H2O) to intricate polymeric structures. Herein, we explore the primary categories of biological molecules:
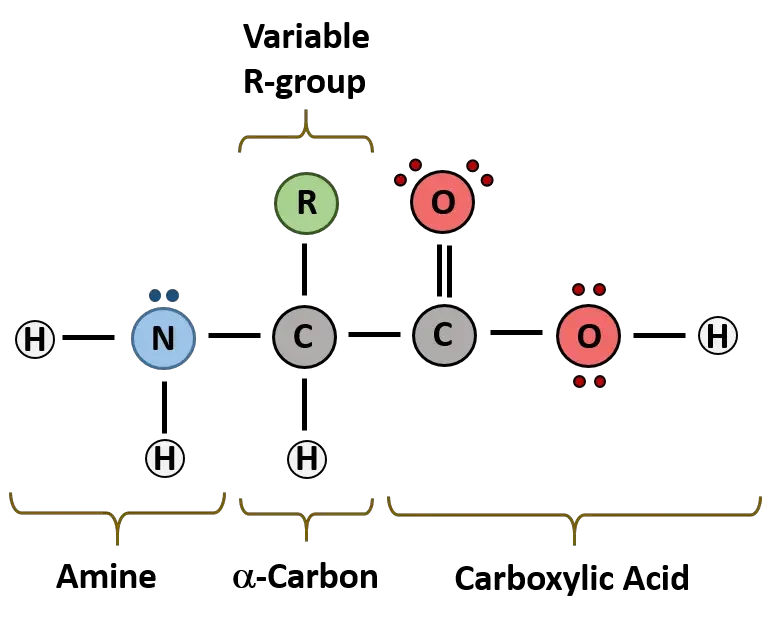
- Proteins:
- Nature and Composition: Proteins are polymeric structures synthesized from monomeric units known as amino acids. These amino acids are encoded by the DNA within cells and assemble in specific sequences to form peptides, which further fold into complex protein structures.
- Functions: Proteins are multifunctional. They act as enzymes catalyzing biochemical reactions, serve as hormones regulating metabolic and growth processes, form antibodies that defend against pathogens, and contribute to the structural framework of organisms.
- Lipids:
- Nature and Composition: Lipids encompass a diverse group of organic compounds, including fats, oils, waxes, and steroids. They are primarily esters of glycerol and fatty acids, characterized by varying chain lengths and saturation levels.
- Amphiphilic Nature: Phospholipids, a subtype of lipids, exhibit both hydrophilic (water-attracting) and hydrophobic (oil-attracting) properties. This dual nature is crucial for forming cellular membranes.
- Carbohydrates:
- Nature and Composition: Carbohydrates are energy reservoirs. They consist of monomeric units called saccharides, which can polymerize to form polysaccharides. While animals primarily utilize glucose for energy, plants produce and store energy in complex carbohydrates like starch.
- Functions: Apart from energy storage, carbohydrates like cellulose provide structural support in plants, forming a vital component of the cell wall.
- Nucleic Acids:
- Nature and Composition: Nucleic acids, namely DNA (Deoxyribonucleic acid) and RNA (Ribonucleic acid), are sequences of nucleotides. These nucleotides comprise nitrogenous bases: adenine (A), thymine (T), guanine (G), and cytosine (C) in DNA.
- Functions: Nucleic acids are the genetic blueprints of life. The specific sequences of the nitrogenous bases in DNA encode information essential for synthesizing proteins and other life processes. This genetic information is transmitted across generations, ensuring the continuity of life.
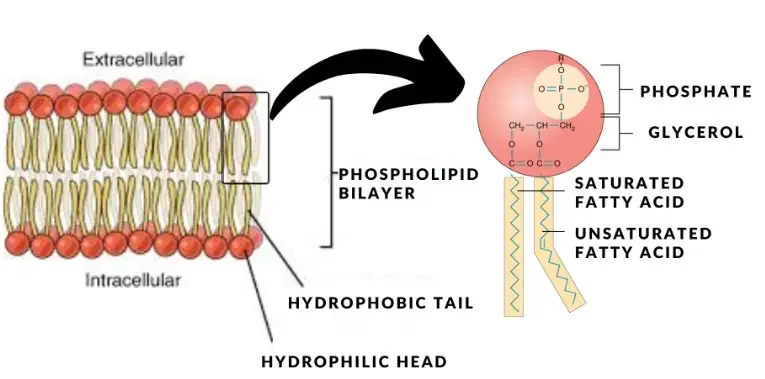
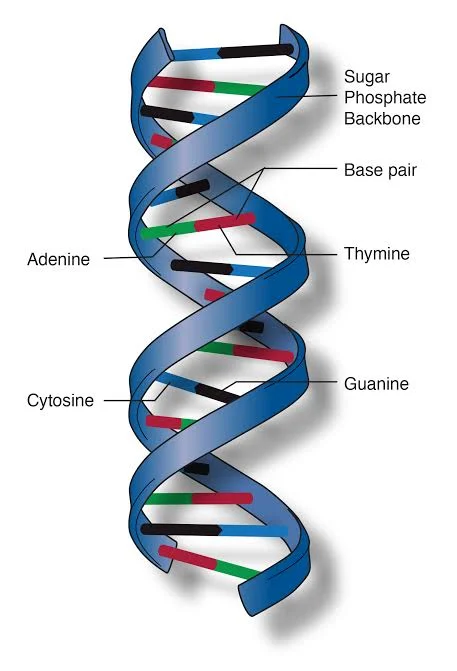
In summation, biological molecules are the cornerstone of life, each playing a distinct role in ensuring the proper functioning and continuity of living organisms. Their intricate structures and diverse functions underscore the complexity and beauty of life at the molecular level.
Classification of Molecules
Molecules, the foundational units in the realm of chemistry, are formed when atoms bond together. These atoms can either be from the same element or from different elements. Based on their composition, molecules can be broadly categorized into two main types: Molecules of Elements and Molecules of Compounds.
- Molecules of Elements:
- Definition: These molecules consist of atoms from a single element chemically bonded together.
- Examples: Oxygen (O2), Nitrogen (N2), and Chlorine (Cl2) are typical examples where two atoms of the same element bond together.
- Atomicity: This term denotes the number of atoms present in a molecule of an element. For instance, the atomicity of oxygen (O2) is 2.
- Sub-Classification Based on Atomicity:
- Monoatomic: Comprising only a single atom, examples include noble gases like Argon and Helium.
- Diatomic: Comprising two atoms, examples include Hydrogen (H2), Oxygen (O2), and Chlorine (Cl2).
- Triatomic: Comprising three atoms of the same element, Ozone (O3) is a classic example.
- Polyatomic: Comprising four or more atoms, examples include certain forms of Boron, Sulphur, and Phosphorus. Fullerene, with a large number of carbon atoms, also falls under this category.
- Molecules of Compounds:
- Definition: These molecules are formed when atoms from different elements bond together, resulting in a compound with distinct properties.
- Examples: Methane (CH4), where one carbon atom bonds with four hydrogen atoms; Ammonia (NH3), where one nitrogen atom bonds with three hydrogen atoms; and Carbon Dioxide (CO2), where one carbon atom bonds with two oxygen atoms.
In essence, the classification of molecules provides a structured way to understand their composition and the nature of the atoms that constitute them. Whether it’s a simple diatomic molecule like Oxygen (O2) or a complex polyatomic molecule like Fullerene, each has its unique structure and properties, playing a crucial role in various chemical and biological processes.
Molecular Bonding
Molecular bonding is a fundamental concept in the realm of chemistry, governing the interactions between atoms and the formation of molecules. The nature of these bonds is determined by the electrons, particularly those in the outermost shell, known as valence electrons. Here, we delve into the intricacies of molecular bonding:
- Atomic Structure:
- Nucleus: At the core of an atom lies the nucleus, housing protons (positively charged) and neutrons (neutral).
- Electron Cloud: Surrounding the nucleus are electrons, negatively charged particles, distributed in specific orbits or shells. The number of protons and electrons in an atom is equal, rendering the atom electrically neutral.
- Valence Electrons and Reactivity:
- Electron Distribution: Electrons are distributed across various orbits, with each orbit having a defined capacity. While inner orbits have electrons strongly attracted to the nucleus, the outermost orbit electrons experience weaker attractions.
- Role in Bonding: The electrons in the outermost shell, or valence electrons, play a pivotal role in bond formation. Their number dictates the atom’s chemical properties and reactivity.
- Types of Bonds:
- Covalent Bonds: When atoms share electrons, they form covalent bonds. Depending on the number of shared electrons, atoms can form single, double, or triple covalent bonds. These bonds are robust, often found in biological molecules, storing energy vital for living organisms. To harness this energy, organisms deploy enzymes to break these bonds.
- Ionic Bonds: At times, atoms transfer electrons. The donor atom becomes a positively charged cation, while the recipient becomes a negatively charged anion. The electrostatic attraction between these oppositely charged ions results in an ionic bond. A classic example is the formation of sodium chloride (NaCl) from sodium (Na+) and chloride (Cl–) ions.
- Physical State of Ionic Compounds:
- Ionic compounds, due to their nature, are typically solid or occasionally liquid at ambient conditions.
In conclusion, molecular bonding is a dynamic interplay of atomic structures and electron interactions. Whether through sharing or transferring electrons, atoms find ways to achieve stability, leading to the diverse array of molecules and compounds we observe in the natural world.
Examples of Molecule
Molecules, particularly those based on carbon, play a pivotal role in the biochemistry of living organisms. Their diverse structures and functionalities underpin the myriad processes that sustain life. Here, we explore two quintessential examples of molecules that have profound biological significance:
- Carbon-Based Molecules:
- Significance of Carbon: Carbon, with its tetravalency, possesses the unique ability to form four covalent bonds. This facilitates the formation of a vast array of molecules, both simple and complex, by associating with elements like hydrogen (H2), oxygen (O2), and nitrogen (N2).
- Evolutionary Perspective: Many evolutionary theories postulate that life’s early emergence on Earth was catalyzed by the synthesis of diverse carbon-based molecules. The inherent flexibility imparted by double bonds between carbon atoms allows these molecules to engage in versatile interactions, such as receptor binding, which is crucial for various biological functions.
- Adenosine Triphosphate (ATP):
- Role in Energy Metabolism: ATP is universally recognized as the primary energy currency of cells. It captures and stores energy during metabolic processes like respiration and glycolysis, where glucose is broken down.
- Molecular Composition: ATP is a complex molecule, comprising elements like carbon, oxygen, phosphorous, and nitrogen. Structurally, it features a cyclic core with a terminal phosphate group (PO4–).
- Energy Release: The hydrolysis of the terminal phosphate bond in ATP releases energy. This energy is harnessed by enzymes to catalyze various biochemical reactions. Following this hydrolysis, ATP is converted to adenosine diphosphate (ADP) and a free phosphate group, awaiting another cycle of energy storage.
In essence, molecules like carbon-based compounds and ATP underscore the intricate and dynamic nature of biological systems. Their roles, from providing structural flexibility to storing and transferring energy, are fundamental to the continuity and vitality of life.
Quiz
What is the smallest unit of a compound that retains the chemical properties of that compound?
a) Atom
b) Electron
c) Proton
d) Molecule
[expand title=”Show answer” swaptitle=”Hide answer”] d) Molecule [/expand]
Which of the following is a diatomic molecule?
a) O2
b) H2O
c) CH4
d) CO2
[expand title=”Show answer” swaptitle=”Hide answer”] a) O2 [/expand]
Which type of bond is formed when atoms share electrons?
a) Ionic bond
b) Covalent bond
c) Metallic bond
d) Hydrogen bond
[expand title=”Show answer” swaptitle=”Hide answer”] b) Covalent bond [/expand]
Which molecule is known as the universal solvent?
a) Oxygen
b) Carbon dioxide
c) Water
d) Methane
[expand title=”Show answer” swaptitle=”Hide answer”] c) Water [/expand]
Which of the following is NOT a molecule?
a) Ne
b) N2
c) O2
d) H2
[expand title=”Show answer” swaptitle=”Hide answer”] a) Ne [/expand]
How many atoms are present in a molecule of H2O?
a) 1
b) 2
c) 3
d) 4
[expand title=”Show answer” swaptitle=”Hide answer”] c) 3 [/expand]
Which molecule is essential for cellular respiration?
a) CO2
b) O2
c) N2
d) CH4
[expand title=”Show answer” swaptitle=”Hide answer”] b) O2 [/expand]
Which of the following molecules is responsible for genetic information in cells?
a) Protein
b) Lipid
c) DNA
d) Carbohydrate
[expand title=”Show answer” swaptitle=”Hide answer”] c) DNA [/expand]
Which molecule is known as the energy currency of the cell?
a) Glucose
b) ATP
c) Amino acid
d) Fatty acid
[expand title=”Show answer” swaptitle=”Hide answer”] b) ATP [/expand]
Which type of bond is formed due to the transfer of electrons from one atom to another?
a) Covalent bond
b) Hydrogen bond
c) Ionic bond
d) Metallic bond
[expand title=”Show answer” swaptitle=”Hide answer”] c) Ionic bond [/expand]
FAQ
What is a molecule?
A molecule is a group of two or more atoms held together by chemical bonds, forming a distinct entity with specific properties.
How do molecules differ from atoms?
Atoms are the basic units of matter, consisting of protons, neutrons, and electrons. Molecules are formed when two or more atoms bond together.
What are the different types of molecular bonds?
The primary types of molecular bonds are covalent (atoms share electrons) and ionic (atoms transfer electrons, leading to attraction between positive and negative ions).
Why is water (H2O) considered a molecule?
Water is considered a molecule because it consists of two hydrogen atoms bonded to one oxygen atom, forming a distinct chemical entity.
Are all molecules visible to the naked eye?
No, most molecules are too small to be seen without the aid of a microscope. However, collections of molecules, like a drop of water, are visible.
How do molecules interact with each other?
Molecules interact through various forces like hydrogen bonding, van der Waals forces, and ionic interactions, depending on their structure and the environment.
What role do molecules play in living organisms?
Molecules are fundamental to life, serving as building blocks (like DNA and proteins), energy sources (like glucose and ATP), and performing countless other functions essential for survival and growth.
Is air composed of molecules?
Yes, air is a mixture of various gaseous molecules, including nitrogen (N2), oxygen (O2), carbon dioxide (CO2), and others.
How are molecules studied in the laboratory?
Molecules are studied using various techniques like spectroscopy, chromatography, and X-ray crystallography, which provide insights into their structure, composition, and interactions.
Can molecules be broken down into simpler components?
Yes, molecules can be broken down into their constituent atoms or smaller molecules through chemical reactions or physical processes like heating.
References
- Edwards, C., Lai, T., Ros, K., Honke, G., Cho, K., & Ji, H. (2022). Translation between molecules and natural language. arXiv preprint arXiv:2204.11817.
- Bader, R. F. (1985). Atoms in molecules. Accounts of chemical research, 18(1), 9-15.
- Li, Y., & Zhao, D. (2013). Basics of Molecular Biology. Molecular Imaging: Fundamentals and Applications, 541–601. https://doi.org/10.1007/978-3-642-34303-2_16.
- Bao, G. (2002). Mechanics of biomolecules. Journal of the Mechanics and Physics of Solids, 50(11), 2237-2274.
- Edwards, C., Lai, T., Ros, K., Honke, G., Cho, K., & Ji, H. (2022). Translation between molecules and natural language. arXiv preprint arXiv:2204.11817.