Micro-RNAs (miRNAs) are a family of non-coding RNAs (ncRNAs) that regulate gene expression post-transcriptionally. Since their discovery in 1993, they have been the topic of extensive research due to their role in numerous crucial biological processes. Unlike other noncoding RNAs, microRNAs are created from transcriptional units that are processed by a particular group of endonucleases.
What is miRNA (microRNA)?
- The miRNA or microRNA are tiny (22 to 25 nucleotides) naturally occurring molecules involved in gene control.
- These are the conserved sequences that have been present in all eukaryotes throughout evolution, but certain miRNAs are also found in viruses.
- During genetic screening, Lee et al. found the first microRNA in worms in 1993.
- Lee et colleagues. discovered miRNA while examining the postembryonic development of the model organism C. elegans.
- At the time, it was thought that the miRNA was exclusive to nematodes.
- Lee and colleagues showed that the lin-4 gene could transcribe into mRNA but could not be translated into protein; instead, it transcribes a short RNA molecule between 22 and 60 nucleotides in length that regulates the lin-14 gene through the antisense RNA mechanism.
- The whole miRNA database is available online in the miRBase database.
- Few miRNAs are present in the intronic or exonic regions. The bulk of miRNAs are located in the intergenic regions and have been conserved throughout evolution.
- Some miRNAs are expressed as polycistronic main transcripts and are positioned in a cluster that is physically adjacent to one another.
- Although some miRNAs have their own promoters and are independently transcribed, termed the mono-cistronic main transcript, others do not.
- Eukaryotic genomes include four distinct types of microRNA based on their location and function:
- Intronic miRNA in coding transcription units
- Exonic miRNA in coding transcription units
- Intronic miRNA in non-coding transcription units
- Exonic miRNA in non-coding transcription units
Properties of miRNA
- Mature microRNAs (miRNAs) are a family of naturally occurring, 21–25 nucleotide-long non-coding RNA molecules.
- MicroRNAs are partly complementary to one or more messenger RNA (mRNA) molecules, and their primary purpose is to inhibit gene expression via translational repression, mRNA cleavage, and deadenylation.
- Lee and colleagues1 originally characterised them in 1993, and the term microRNA was coined in 20012.
- Thousands of microRNAs have been found in numerous organisms using random cloning, sequencing, and computational prediction.
- The Sanger Institute hosts the miRBase, which includes information on miRNA nomenclature, sequence data, annotation, and target prediction.
- Mutations are capable of damaging microRNA genes in cancer cells.
- A mutation in a microRNA gene can render a cell devoid of or severely depleted in a certain microRNA.
- Abnormally low levels of a microRNA can result in the overexpression of genes that it regulates, which can promote the development and progression of cancer. A specific microRNA can affect hundreds or thousands of genes by binding to their mRNA.
- The abnormal expression of microRNA has been linked to a variety of human malignancies.
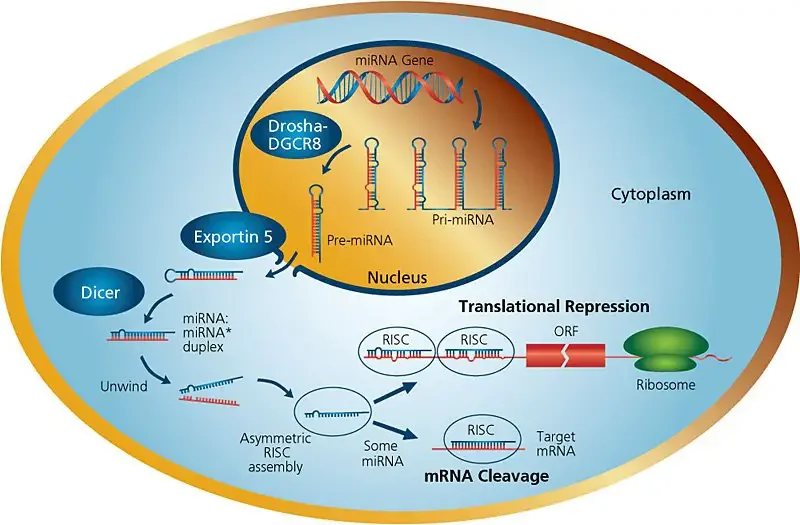
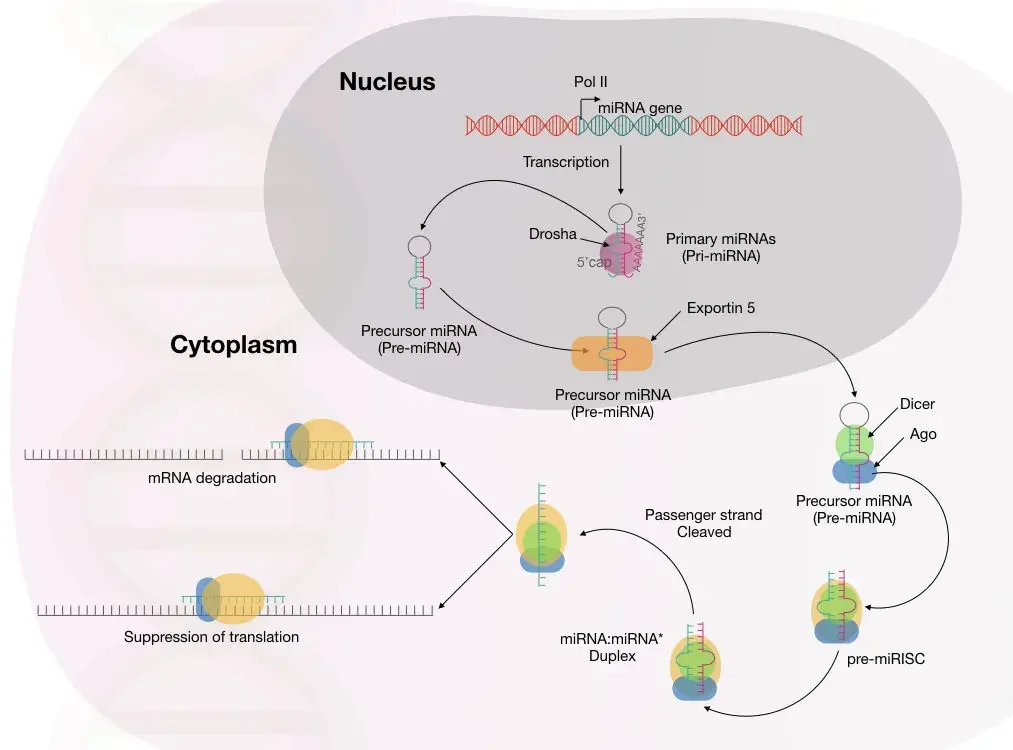
- The first gene transcript is referred to as primary miRNA (pri-miRNA). In the nucleus of the cell, these hairpin-loop molecules are snipped to generate double-stranded precursor miRNA (pre-miRNA)
- The transfer of pre-miRNA to the cytoplasm. There, it is further cleaved to produce a mature, functioning miRNA (mature miRNA molecules are about 22 nucleotides long)
- The mature miRNA initially binds with the RNA interference silencing complex, or RISC.
- The miRNA then attaches to its target messenger RNA (mRNA), inhibiting its translation or triggering its destruction.
Function of miRNA
- miRNAs are primarily involved in gene control. miRNAs can be complementary to several mRNAs. In animals, miRNAs are compatible with the 3′ untranslated regions, but in plants they are complementary to the coding regions.
- In plants, nearly perfect base pairing triggers the cleavage of mRNA. It is partly complementary to mRNA in animals and suppresses both mRNA translation and gene product synthesis. It can also stimulate mRNA deadenylation, resulting in mRNA destabilisation.
- Methylation of DNA at the promoter region can also influence the expression of genes by miRNA. It can also result in changes to histones.
- miRNAs are preserved over the course of evolution and act as excellent phylogenetic markers. A miRNA can target many genes in an organism. Because they are highly conserved, miRNAs are essential for the correct operation of a cell and gene control.
- It has also been discovered that microRNA influence the blood coagulation cascade and hemostasis. In addition to regulating obesity, diabetes, and insulin resistance, microRNAs also regulate obesity, diabetes, and insulin resistance. A specific class of microRNA is discovered to accumulate with age and to produce insulin resistance, resulting in obesity and diabetes.
- They regulate genes involved in the cell cycle, cell signalling, apoptosis, nervous system development, etc., and are responsible for the altered gene expression in addiction.
- It has been discovered that several diseases, including Alzheimer’s, schizophrenia, depression, and anxiety disorders, have altered miRNA expression. It regulates cholesterol metabolism and is involved in cardiomyopathies during cardiovascular disease and stroke.
- Multiple genetic disorders, such as cataract, progressive hearing loss, skeletal and growth abnormalities, etc., are connected with miRNA dysregulation. The deregulation of miRNA has been linked to chronic lymphocytic leukaemia, a kind of malignancy. Moreover, it is connected with colorectal cancer. Hepatocellular carcinoma cell proliferation is correlated with the interaction of microRNA with the gene that suppresses tumour growth.
- miRNAs are also known to inhibit tumour growth. miRNAs can be utilised as targets or instruments for the treatment of a variety of malignancies. Several microRNAs control genes involved in DNA repair. Deficiencies in the DNA repair system result in the accumulation of mutations and the development of cancer. The aberrant expression of DNA-repair-involved microRNA genes has been linked to the development of several forms of cancer.
- MicroRNAs serve a crucial function in the regulation of viral and host genes for the advantage of the virus. It is essential to the pathophysiology of the viral illness.
Disease involve with miRNA
MiRNA is implicated in the proper function of eukaryotic cells, and its dysregulation has been linked to illness. miR2Disease is a manually maintained, publicly accessible database that documents the known associations between miRNA dysregulation and human disease.
Inherited diseases
- A mutation in the seed area of miR-96 causes gradual hereditary hearing loss.
- Hereditary keratoconus with anterior polar cataract is caused by a mutation in the seed region of miR-184.
- The absence of the miR-1792 cluster results in skeletal and growth abnormalities.
Cancer
- Chronic lymphocytic leukaemia was the first disease recognised to be associated with miRNA dysregulation in humans.
- Numerous more miRNAs have ties to cancer and are therefore frequently referred to as “oncomirs.”
- In malignant B cells, microRNAs are involved in key B cell growth pathways such as B-cell receptor (BCR) signalling, B-cell migration/adhesion, cell-cell interactions in immunological niches, and the synthesis and class-switching of immunoglobulins.
- MiRNAs affect the development of B cells, as well as the production of pre-, marginal zone, follicular, B1, plasma, and memory B cells.
- The expression level of miRNAs in tumours is also used for prognosis. Low miR-324a levels in NSCLC tissues may be indicative of poor survival. Either high miR-185 or low miR-133b levels may associated with colorectal cancer metastases and poor survival.
DNA repair and cancer
- Numerous miRNAs can directly target and inhibit genes involved in the cell cycle to regulate cell growth.
- By fixing the faulty miRNA pathway in cancers, a novel technique for treating malignancies is to decrease tumour cell proliferation.
- Cancer is produced by the accumulation of mutations resulting from DNA damage or unrepaired replication mistakes.
- Defects in DNA repair lead to the accumulation of mutations, which can result in the development of cancer.
- Several DNA-repair-related genes are controlled by microRNAs.
Heart disease
- The overall role of miRNA function in the heart has been investigated by blocking miRNA maturation in the mouse heart under specific conditions. This demonstrated that miRNAs are fundamental to its growth.
- Studies of miRNA expression profiling indicate that the expression levels of particular miRNAs are altered in diseased human hearts, suggesting their role in cardiomyopathies.
Hemostasis
- In addition, microRNAs serve key roles in the control of complicated enzyme cascades, such as the blood coagulation system.
- Recent large-scale investigations of functional microRNA targeting have revealed reasonable therapeutic targets in the hemostatic system.
Obesity
- miRNAs play critical functions in regulating the differentiation of stem cell progenitors into adipocytes.
- On the immortalised human bone marrow-derived stromal cell line hMSC-Tert20, studies to establish the involvement of pluripotent stem cells in adipogenesis were conducted.
- During the adipogenic programming of both immortalised and primary hMSCs, decreased expression of miR-155, miR-221, and miR222 suggests that they function as negative regulators of differentiation.
- In contrast, overexpression of miRNAs 155, 221, and 222 dramatically decreased adipogenesis and restricted the activation of the master regulators PPAR and CCAAT/enhancer-binding protein alpha (CEBPA). This paves the road for potential obesity treatments based on genetics.
Other disease
- miRNAs responsible for several illnesses of the Nervous System.
- Also responsible for Kidney illness is miRNA.
miRNA Biogenesis
- The miRNA-encoding genes are significantly longer than the mature miRNA molecule.
- Numerous miRNAs are known to exist in the introns of their pre-mRNA host genes and to share regulatory elements, primary transcripts, and expression profiles.
- Few primary transcripts have been completely found for the remaining miRNA genes that are transcribed from their own promoters.
- MicroRNAs are transcribed by RNA polymerase II as pri-miRNAs, which are giant RNA precursors with a 5′ cap and poly-A tail.
- The microprocessor complex, composed of the RNase III enzyme Drosha and the double-stranded RNA-binding protein Pasha/DGCR85, processes pri-miRNAs in the nucleus.
- The resultant pre-miRNAs are roughly 70 nucleotides in length and have defective stem-loop structures.
- The karyopherin exportin 5 (Exp5) and Ran-GTP complex then exports the pre-miRNAs into the cytoplasm.
- Ran (ras-related nuclear protein) is a tiny GTP-binding protein that is important for the translocation of RNA and proteins via the nuclear pore complex and is a member of the RAS superfamily.
- Ran GTPase binds Exp5 and forms a heterotrimer with pre-miRNAs in the nucleus.
- Once in the cytoplasm, the pre-miRNAs are further processed by the RNAse III enzyme Dicer to produce the miRNA, a double-stranded RNA of around 22 nucleotides.
- Dicer also triggers the creation of the RNA-induced silencing complex (RISC). RISC is responsible for the observed gene silencing resulting from miRNA production and RNA interference.
Function of Dicer
- Dicers are large 200 kDa proteins that contain an ATPase/RNA helicase, a DUF283 (Domain of unknown function) domain, a PAZ (Piwi, Argonaut and Zwille) domain that can bind the characteristic two-base 3′ overhangs of miRNA and siRNA, two catalytic RNase III domains (RIIIa and RIIIb), and a C-terminal double-stranded RNA- (dsRBD).
- Dicer acts as a monomer and contains a single processing core, with the two RNase III domains dimerizing intramolecularly.
- Each RNase domain breaks one RNA strand of the duplex independently to yield products with 2-nt 3′ overhangs.
- Dicer enzymes also convert dsRNA to siRNAs in addition to removing miRNAs from pre-miRNAs.
- The miRNA pathway resembles the central phases of RNA interference (RNAi) in animals after Dicer breakage.
- In contrast to siRNAs, microRNAs can drive RISC to down-regulate gene expression via translational repression (due to lesser complementarity between miRNA and mRNA) or mediate mRNA cleavage like siRNAs do.
- The selection of post-transcriptional processes is not dependent on the kind of silencing RNA (siRNA or miRNA), but rather on the degree of complementarity.
- If the miRNA is exactly or nearly perfectly complementary to its target, it can cleave the target mRNA precisely.
- Typically, endogenously produced microRNAs are imperfectly complementary to their target gene(s) and influence gene expression by inhibiting translation.
RISC Complex
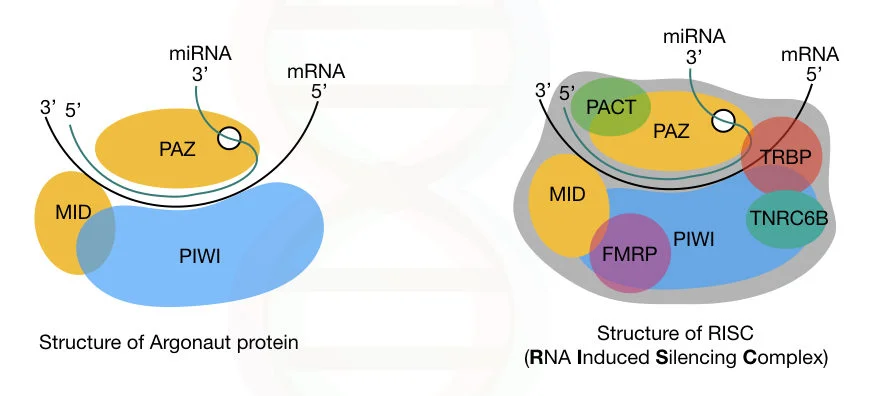
- Two complementary short RNA molecules are produced when Dicer cleaves the pre-miRNA stem-loop, but only one is integrated into the RISC complex.
- RISC is a ribonucleoprotein complex containing Argonaute (Ago) protein family members.
- The endonuclease activity of Argonaute proteins is directed against mRNA strands that are complementary to their associated miRNA fragment.
- Additionally, Argonaut is partially accountable for the selection of the guide strand and the elimination of the passenger strand.
- The guide strand is picked by the argonaute protein based on the stability of the 5′ end of the integrated strand.
- As a RISC complex substrate, the remaining strand, called as the passenger strand (*), is destroyed.
- Selection of the guide strand from the double-stranded RNA appears to be mostly dependent on the stability of the two ends of the dsRNA.
- The strand with the less stable base pairing of the 2–4 nucleotides at the 5′ end of the duplex preferentially connects with RISC, so becoming the active miRNA.
- miRNAs exert their regulatory effects by binding to imperfect complementary sites within the 3′ untranslated regions (UTRs) of their mRNA targets, following integration into an active RISC complex.
- The creation of double-stranded RNA, which results from the binding of the microRNA, inhibits translation.
Applications of microRNA
- Assays and investigations of function gain and loss.
- Specific miRNA identification and target-gene connection investigations.
- Evaluation of gene expression and gene silencing.
- Studies on cancer and viral infections.
- Other therapeutic methods such as cancer diagnosis, prognosis, and development monitoring.
- In addition, it is utilised in the research of cell metabolism, cell proliferation, and death.
References
- Kozlowski, Piotr & Starega-Roslan, Julia & Legacz, Marta & Magnus, Marcin & Krzyzosiak, Wlodzimierz. (2008). Structures of MicroRNA Precursors. 10.1007/978-1-4020-8533-8_1.
- Pogue AI, Clement C, Hill JM, Lukiw WJ. Evolution of microRNA (miRNA) Structure and Function in Plants and Animals: Relevance to Aging and Disease. J Aging Sci. 2014 Jun;2(2):119. doi: 10.4172/2329-8847.1000119. PMID: 26146648; PMCID: PMC4489142.
- Macfarlane LA, Murphy PR. MicroRNA: Biogenesis, Function and Role in Cancer. Curr Genomics. 2010 Nov;11(7):537-61. doi: 10.2174/138920210793175895. PMID: 21532838; PMCID: PMC3048316.
- https://www.jbc.org/article/S0021-9258(20)72693-1/fulltext
- https://www.sigmaaldrich.com/IN/en/technical-documents/technical-article/genomics/gene-expression-and-silencing/mirna-introduction
- https://link.springer.com/protocol/10.1007/978-1-60761-811-9_17
- https://cancer.osu.edu/microrna
- https://www.frontiersin.org/articles/10.3389/fendo.2018.00402/full#:~:text=miRNAs%20are%20small%20non%2Dcoding,to%20suppress%20expression%20(14).
- https://tools4mirs.org/software/precursor_prediction/
- https://geneticeducation.co.in/microrna-mirna-and-gene-regulation/