What is Middlebrook Agar?
- Middlebrook Agar is a type of culture medium that was developed by Dubos and Middlebrook for the cultivation of mycobacteria, specifically the tubercle bacilli. It is formulated with key ingredients like oleic acid and albumin, which serve to protect the mycobacteria from toxic agents and promote their growth. This agar base was further improved by Middlebrook and Cohn, resulting in the formulation known as Middlebrook 7H10 Agar Base.
- Middlebrook 7H10 Agar Base has been found to support rapid and luxuriant growth of various Mycobacterium species. It is commonly used for the isolation, cultivation, and sensitivity testing of M. tuberculosis when enriched with OADC (oleic acid, albumin, dextrose, and catalase) Growth Supplement and glycerol.
- One of the advantages of Middlebrook agar is that it tends to have fewer contaminants compared to egg-based media traditionally used for mycobacterial cultivation. This makes it a preferred choice in laboratory settings for the cultivation of mycobacteria.
- The composition of Middlebrook media includes several inorganic salts that facilitate the growth of mycobacteria. Sodium citrate helps retain inorganic cations in solution, while glycerol serves as a carbon and energy source. The OADC Growth Supplement contains oleic acid, bovine albumin, sodium chloride, dextrose, and catalase. Oleic acid and other long-chain fatty acids are essential for the metabolism of mycobacteria. Albumin binds to potentially toxic fatty acids, preventing their harmful effects on the mycobacteria. Dextrose provides a source of energy, and catalase neutralizes toxic peroxides. Additionally, malachite green, present in the medium, partially inhibits the growth of other bacteria.
- It is important to note that mycobacteria are strict aerobes, requiring increased CO2 tension and aerobic conditions during incubation. Proper decontamination of specimens and careful handling of samples are crucial to avoid contamination. When collecting specimens, sputum should be preferred over saliva to ensure accurate results.
- In summary, Middlebrook Agar, particularly Middlebrook 7H10 Agar Base, is a specialized culture medium designed for the cultivation and study of mycobacteria. It provides optimal conditions for the growth of these bacteria while minimizing contamination, making it a valuable tool in microbiology laboratories.
Principle of Middlebrook Agar
The principle of Middlebrook Agar lies in its composition, which provides essential substances for the growth and metabolism of mycobacteria. The agar contains a variety of inorganic salts that play a crucial role in supporting the growth of these bacteria.
One of the key components of Middlebrook Agar is sodium citrate. When converted to citric acid, it helps in retaining certain inorganic cations in solution, ensuring their availability for the mycobacteria.
Glycerol, another important ingredient, serves as an abundant source of carbon and energy for the mycobacteria. It provides the necessary nutrients for their growth and metabolic activities.
Sodium chloride, present in the enriched medium, acts as an electrolyte and helps maintain osmotic equilibrium. It is essential for the proper functioning of the mycobacterial cells.
Oleic acid, along with other long-chain fatty acids, is a crucial component that can be utilized by tubercle bacilli. These fatty acids play a significant role in the metabolism of mycobacteria, supporting their growth and development.
The albumin present in the Middlebrook Agar serves a protective role for the tubercle bacilli. It helps in safeguarding them against toxic agents, thereby enhancing their recovery during the initial isolation process.
Dextrose, a fermentable carbohydrate and an energy source, provides the mycobacteria with the necessary fuel for their metabolic activities and growth.
Catalase, another component of Middlebrook Agar, acts to destroy toxic peroxides that may be present in the medium. This helps in maintaining a favorable environment for the growth of mycobacteria.
Additionally, the presence of malachite green dye in the agar contributes to the partial inhibition of other bacteria. This dye helps control the growth of contaminating organisms, ensuring a selective environment that promotes the growth of mycobacteria.
In summary, the principle of Middlebrook Agar revolves around its composition, which includes inorganic salts, glycerol, oleic acid, albumin, dextrose, catalase, and malachite green dye. These components provide essential nutrients, protect against toxic agents, and create a selective environment that supports the growth and recovery of mycobacteria.
Composition of Middlebrook Agar
| Ingredients | Gms/Litre |
|---|---|
| Ammonium sulphate | 0.500 |
| L-Glutamic acid | 0.500 |
| Monopotassium phosphate | 1.500 |
| Disodium phosphate | 1.500 |
| Sodium citrate | 0.400 |
| Ferric ammonium citrate | 0.040 |
| Magnesium sulphate | 0.025 |
| Calcium chloride | 0.0005 |
| Zinc sulphate | 0.001 |
| Copper sulphate | 0.001 |
| Pyridoxine hydrochloride | 0.001 |
| Biotin | 0.0005 |
| Malachite green | 0.00025 |
| Agar | 15.000 |
| Final pH (at 25°C) | 6.6±0.2 |
| Formula adjusted | Standardized to suit performance parameters |
Preparation of Middlebrook Agar
The preparation of Middlebrook Agar involves the following steps:
- Suspend 9.73 grams of Middlebrook Agar in 450 ml of distilled water. To this mixture, add 2.5 ml of glycerol. The glycerol serves as an additional component to enhance the growth of mycobacteria.
- Heat the suspension to boiling while stirring to ensure complete dissolution of the medium. The boiling step helps in dissolving all the ingredients uniformly.
- Sterilize the medium at 15 pounds of pressure (121°C) for 10 minutes using an autoclave. This sterilization process ensures that any contaminants present in the medium are eliminated.
- After sterilization, allow the medium to cool to a temperature of 45-50°C. It is important to cool the medium to this range to prevent any heat damage to the mycobacteria when they are inoculated.
- Aseptically add 50 ml of Middlebrook OADC Growth Supplement (FD018) to the cooled agar. The OADC Growth Supplement contains additional components necessary for the optimal growth and recovery of mycobacteria.
- Thoroughly mix the agar and the OADC Growth Supplement to ensure even distribution of the supplement throughout the medium.
- Pour the prepared agar into sterile screw-capped tubes or containers. Ensure that the containers are sterile to avoid any contamination.
- It is recommended to store the prepared medium in the dark both before and after inoculation. This helps maintain the stability of the medium and prevents any potential light-induced changes.
Following these steps ensures the proper preparation of Middlebrook Agar, providing a suitable medium for the cultivation and study of mycobacteria.
Test Procedure on Middlebrook Agar – Method of Use
The test procedure on Middlebrook Agar for the cultivation of acid-fast cultures and specimens, such as Mycobacterium marinum or Mycobacterium ulcerans, involves the following steps:
- Ensure adherence to established laboratory safety procedures when working with acid-fast cultures and specimens. Consult appropriate references for detailed procedural information on specimen processing and media inoculation.
- Inoculate two sets of media with the specimens obtained from skin or soft tissue suspected of containing Mycobacterium marinum or Mycobacterium ulcerans. Incubate one set at 35-37°C and the other set at room temperature.
- Using a Pasteur pipette, inoculate Middlebrook 7H11 Agar with 1-2 drops of decontaminated, concentrated specimen. Carefully place the drops onto the agar surface.
- Allow the inoculated media to remain at room temperature for several hours, if possible, until the inoculum dries or is absorbed by the agar.
- Incubate the agar plates at 35-37°C in an atmosphere of 5-10% CO2. Protect the plates from light during incubation. It is recommended to incubate plates agar-side down to ensure proper absorption of the inoculum. If using gas-permeable bags, place one plate per bag to allow adequate gas exchange.
- Incubate tubed media in a slanted position with caps loosened for the first week. This allows for the circulation of CO2 and absorption of the inoculum into the media. After the first week or two, tighten the caps to prevent dehydration of the media.
- Examine the cultures within 5-7 days after inoculation and once a week thereafter for a minimum of 8 weeks. Additional incubation may be necessary, up to 10-12 weeks or more, in selected cases or if the original smear was positive and the culture remains negative at 8 weeks.
- Monitor the cultures for growth rate, pigment production, and colony morphology.
- If growth is detected, stain the colonies using an acid-fast staining method to confirm that the isolate is acid-fast.
- Subculture the acid-fast colonies onto an appropriate medium for further identification and characterization. Follow established laboratory procedures and consult appropriate references for detailed instructions, if necessary.
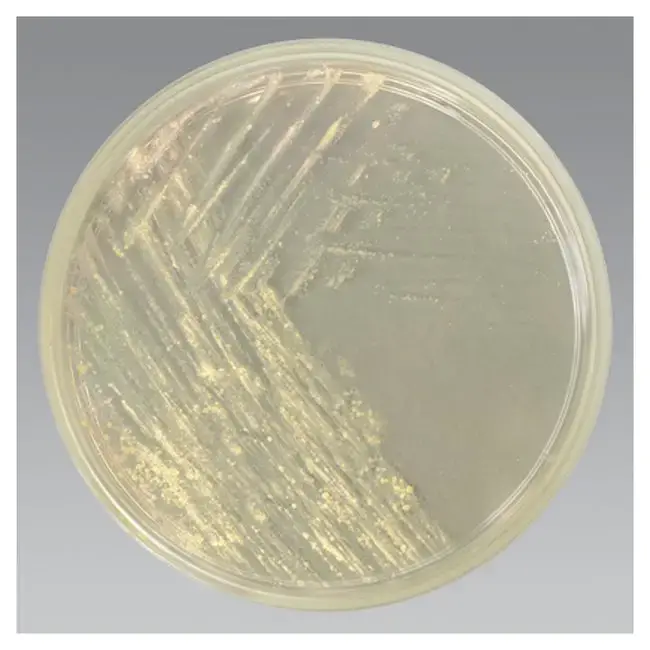
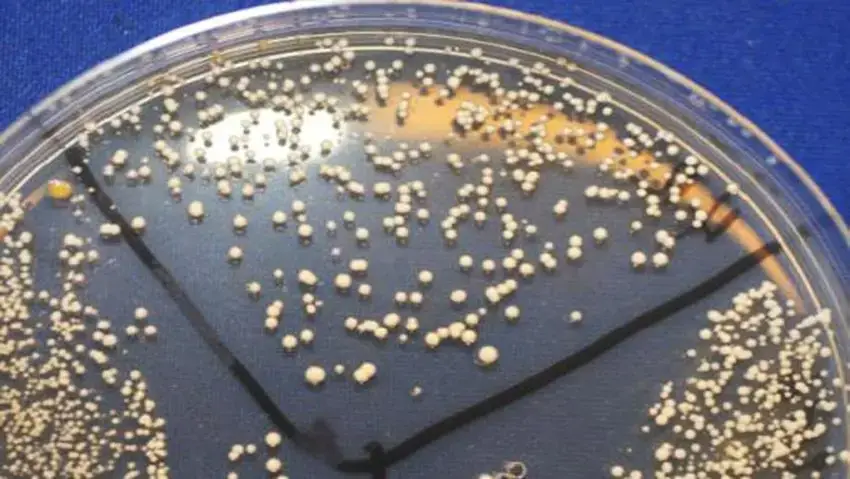
By following these test procedures, Middlebrook Agar can be effectively utilized for the cultivation, growth monitoring, and confirmation of acid-fast organisms such as Mycobacterium marinum or Mycobacterium ulcerans.
Results and Interpretation on Middlebrook Agar
Results and interpretation on Middlebrook Agar are as follows:
- Reading Time: Cultures should be observed and read within 5-7 days after inoculation and then once a week for up to 8 weeks. This allows sufficient time for the growth and development of mycobacterial colonies.
- Observation Method: Invert the plates or bottles on the stage of a dissecting microscope for observation. Use transmitted light and adjust the magnification between 10-60× for optimal viewing.
- Rapid Scanning: Begin by scanning the agar plates or bottles at a lower magnification (10-20×) to quickly identify the presence of colonies. This helps in getting an initial overview of the culture.
- Colony Morphology: For a more detailed examination, switch to higher magnification (30-60×). This allows for the observation of colony morphology, particularly the presence of serpentine cord-like colonies. Note any specific characteristics of the colonies.
- Record Observations: Make detailed observations and record the following:
- a. Time for Colony Visibility: Note the number of days it takes for colonies to become macroscopically visible. This provides information about the growth rate of the mycobacteria.
- b. Colony Count: Count the number of colonies present on the plates or bottles. The count can be categorized as follows:
- No colonies: Indicates a negative result.Less than 50 colonies: Record the actual count.50-100 colonies: Categorized as 1+.100-200 colonies: Categorized as 2+.Almost confluent (200-500 colonies): Categorized as 3+.Confluent (more than 500 colonies): Categorized as 4+.
- White, cream, or buff: Indicates nonchromogenic (NC) mycobacteria.
- Lemon, yellow, orange, red: Indicates chromogenic (Ch) mycobacteria.
By recording these observations, a comprehensive interpretation of the culture on Middlebrook Agar can be made, providing valuable information about the growth, colony count, and pigmentation of the mycobacterial species under study.
Quality Control
The quality control of Middlebrook Agar involves assessing various parameters to ensure its performance and reliability. The following information provides details on the quality control measures for Middlebrook Agar:
- Appearance: Middlebrook Agar should have a light yellow to light green homogeneous free-flowing powder appearance. This indicates the proper physical characteristics of the agar.
- Gelling: When prepared, the agar should form a firm gel that is comparable to a 1.5% Agar gel. This indicates the appropriate gelling properties of the agar.
- Colour and Clarity of Prepared Medium: The prepared agar should exhibit a light amber color, appearing clear to slightly opalescent with a greenish tinge in Petri plates. This ensures that the medium has the expected visual appearance.
- Reaction: A 1.95% w/v aqueous solution of the agar, containing glycerol, should exhibit a pH of 6.6±0.2 when measured at 25°C. This confirms the desired pH range for the agar.
- pH: The pH of the prepared agar should fall within the range of 6.40-6.80. This ensures that the pH is within the specified limits for optimal performance.
- Cultural Response: The cultural response of Middlebrook Agar is evaluated using specific organisms. When Middlebrook OADC Growth Supplement (FD018) and glycerol are added to the agar, and the agar is incubated at 35-37°C for 2-4 weeks, the following organisms should exhibit good-luxuriant growth:
- Mycobacterium fortuitum ATCC 6841
- Mycobacterium smegmatis ATCC 14468
- Mycobacterium tuberculosis H37RV (25618)
The growth of these organisms confirms the suitability and effectiveness of the agar for supporting the growth of mycobacteria.
By conducting these quality control tests, the consistency and performance of Middlebrook Agar can be ensured, providing reliable results for microbiological applications involving mycobacterial cultures.
Uses of Middlebrook Agar
Middlebrook Agar, specifically Middlebrook 7H10 Agar supplemented with Middlebrook OADC Enrichment, has various uses in the field of microbiology. These include:
- Isolation and Cultivation of Mycobacteria: Middlebrook Agar is commonly used for the qualitative isolation and cultivation of mycobacteria. It provides a selective and supportive environment for the growth of these bacteria, allowing for their isolation from clinical specimens or environmental samples.
- Qualitative Procedures: Middlebrook 7H10 Agar, when supplemented with Middlebrook OADC Enrichment, is particularly suitable for qualitative procedures in the identification and characterization of mycobacteria. It enables the differentiation of different mycobacterial species based on their growth characteristics and colony morphology.
- Sensitivity Testing of M. tuberculosis: Middlebrook Agar is widely employed for the sensitivity testing of Mycobacterium tuberculosis. This testing involves assessing the susceptibility of M. tuberculosis strains to various antibiotics or anti-tuberculosis drugs. By determining the effectiveness of different drugs, it helps in guiding the appropriate treatment strategies for tuberculosis.
- Diagnostic Procedures: Middlebrook Agar is utilized in diagnostic laboratories for the primary isolation and cultivation of Mycobacterium tuberculosis. By providing an optimal growth medium, it aids in the detection and identification of this pathogenic bacterium, which is responsible for causing tuberculosis in humans.
In summary, Middlebrook Agar, specifically Middlebrook 7H10 Agar supplemented with Middlebrook OADC Enrichment, finds application in qualitative procedures, isolation, cultivation, and sensitivity testing of Mycobacterium tuberculosis. Its selective properties and ability to support the growth of mycobacteria make it a valuable tool in microbiology laboratories for diagnosing and studying tuberculosis.
Limitations of Middlebrook Agar
Middlebrook Agar, despite its usefulness, has several limitations that should be taken into consideration. These limitations include:
- Identification Limitations: While Middlebrook Agar provides a suitable medium for the isolation and cultivation of mycobacteria, it does not provide complete identification of the isolated colonies. Further testing using biochemical, immunological, molecular, or mass spectrometry methods is required for accurate identification of the mycobacterial species.
- Partial Selectivity: Middlebrook Agar is only partially selective for mycobacteria. If specimens are not appropriately pretreated for decontamination, other bacteria may also grow on the agar, leading to potential interference with the growth and identification of mycobacteria.
- Aerobic Growth and CO2 Requirement: Mycobacteria are strict aerobes and their growth is stimulated by increased levels of CO2. To recover mycobacteria effectively, the screw caps on tubes or bottles must be handled as directed to allow proper exchange of CO2. Incubation in a 5-10% CO2 atmosphere is required for optimal recovery of mycobacteria. Candle extinction jars, which are commonly used for creating a CO2-enriched atmosphere, may not support the growth of mycobacteria well, although the reasons for this are not fully understood.
- Light and Heat Sensitivity: Inoculated Middlebrook Agar should be protected from exposure to light or excessive heat. Exposure to light or high temperatures can lead to the release of formaldehyde in the agar, which may inhibit or kill mycobacteria, affecting their growth and recovery.
- Limitations in Culture Results: Negative culture results on Middlebrook Agar do not rule out an active mycobacterial infection. There are various factors that can contribute to unsuccessful cultures, including the use of non-representative specimens (e.g., saliva instead of sputum), destruction of mycobacteria during specimen processing, and interference from gross contamination. Additionally, if proper aerobic conditions and increased CO2 tension are not provided during incubation, it can impact the growth of mycobacteria and lead to suboptimal results.
It is important to consider these limitations when using Middlebrook Agar and to supplement the agar with appropriate testing methods to ensure accurate identification of mycobacteria and reliable culture results.
FAQ
What is Middlebrook Agar?
Middlebrook Agar is a specialized culture medium used for the isolation, cultivation, and study of mycobacteria, particularly Mycobacterium tuberculosis.
What are the key ingredients in Middlebrook Agar?
Middlebrook Agar contains inorganic salts, oleic acid, albumin, glycerol, and other components necessary for the growth and metabolism of mycobacteria.
How is Middlebrook Agar prepared?
Middlebrook Agar is prepared by dissolving the agar powder in distilled water, sterilizing the medium, adding supplements such as Middlebrook OADC Growth Supplement, and pouring the agar into sterile containers.
What is the role of Middlebrook OADC Growth Supplement?
Middlebrook OADC Growth Supplement provides additional nutrients and growth factors necessary for optimal growth and recovery of mycobacteria.
How long does it take for mycobacteria to grow on Middlebrook Agar?
Mycobacteria usually require 5-7 days to become macroscopically visible on Middlebrook Agar. Subsequent readings can be performed weekly for up to 8 weeks.
Is Middlebrook Agar selective for mycobacteria?
Middlebrook Agar is partially selective for mycobacteria. However, if specimens are not appropriately pretreated for decontamination, other bacteria may also grow on the agar.
What are the limitations of Middlebrook Agar?
Some limitations of Middlebrook Agar include the need for additional testing for complete identification of mycobacteria, the requirement for proper decontamination of specimens, and the importance of providing adequate aerobic conditions and increased CO2 tension during incubation.
Can Middlebrook Agar be used for sensitivity testing of Mycobacterium tuberculosis?
Yes, Middlebrook Agar can be used for sensitivity testing of M. tuberculosis to determine its susceptibility to different antibiotics or anti-tuberculosis drugs.
How should Middlebrook Agar be stored?
Middlebrook Agar should be stored in a dark environment both before and after inoculation to maintain its stability. It should also be protected from light and excessive heat, as exposure can release formaldehyde, which may inhibit or kill mycobacteria.
Are negative culture results on Middlebrook Agar conclusive?
Negative culture results on Middlebrook Agar do not rule out an active mycobacterial infection. Various factors can contribute to unsuccessful cultures, such as non-representative specimens, destruction of mycobacteria during specimen processing, or gross contamination. Additional testing and clinical evaluation may be necessary in such cases.
References
- https://www.bd.com/resource.aspx?IDX=9004
- https://hardydiagnostics.com/c6283
- https://bioactiva.com/pub/media/sebwite/productdownloads//m/i/middlebrook-7h10-agar-plate.pdf
- https://assets.fishersci.com/TFS-Assets/LSG/manuals/IFU1605.pdf
- https://www.sigmaaldrich.com/IN/en/product/sial/m0303
- https://www.thermofisher.com/order/catalog/product/R01600
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.