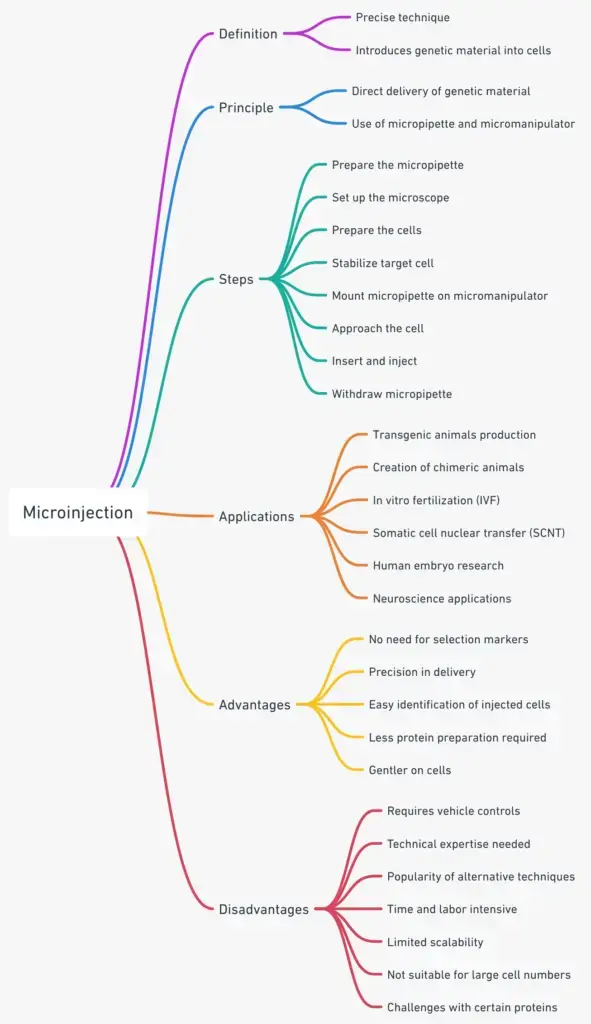
What is Microinjection?
- Microinjection is a sophisticated technique employed in the realm of molecular biology and genetics. It involves the direct introduction of DNA or other genetic materials into a cell. This is achieved using a micropipette or a fine glass needle, specifically designed for this purpose.
- The primary advantage of microinjection is its ability to facilitate the efficient transfer and subsequent integration of desired genes into the host cell’s genome. This method offers unparalleled precision in the delivery of genetic materials, ensuring that the desired outcome is achieved with minimal external interference. Such precision has rendered microinjection an invaluable tool in a plethora of research domains.
- The inception of DNA microinjection can be traced back to the pioneering work of Dr. Marshall A. Barber in the early 19th century. His groundbreaking concept laid the foundation for subsequent advancements in this technique. Over the years, microinjection has undergone significant refinements, adapting to the rapid progress in the biomedical sciences.
- Today, the applications of microinjection span a wide spectrum of scientific endeavors. It plays a pivotal role in the creation of transgenic organisms, facilitating animal cloning processes, and aiding in treatments related to human infertility. Furthermore, its contributions to genetic engineering and genome editing have been instrumental in pushing the boundaries of what is scientifically achievable.
- In conclusion, microinjection stands as a testament to the confluence of precision, innovation, and scientific rigor. Its continued relevance in modern research underscores its significance in the ever-evolving landscape of molecular biology and genetics.
Definition of Microinjection
Microinjection is a precise technique used to introduce DNA or other genetic materials directly into a cell using a micropipette or fine glass needle. It is widely employed in genetic engineering, genome editing, and various biomedical research areas.
Principle of Microinjection
Microinjection operates on the fundamental principle of directly delivering genetic material into specific cells. This is achieved using a fine glass needle, known as a micropipette. To ensure accuracy during this process, two essential tools are employed: a micromanipulator and a microinjector.
Here’s a step-by-step breakdown of the principle:
- Visualization: The entire process is closely monitored under a high-resolution microscope. This ensures that the target cell is clearly visible and accessible.
- Micropipette: This fine glass needle holds the genetic material in a fluid. It’s designed to be extremely thin, ensuring minimal disruption to the cell during insertion.
- Micromanipulator: This device allows for controlled and precise movements of the micropipette. It ensures that the genetic material is delivered to the exact location within the cell.
- Microinjector: By applying a specific hydrostatic pressure, the microinjector releases the fluid containing the DNA from the micropipette into the cell.
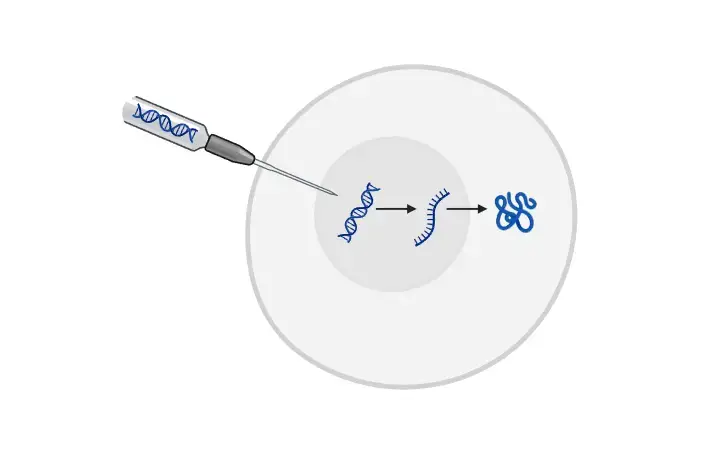
Types of Microinjection
Microinjection, as a technique, can be categorized based on the target cell or tissue and the purpose of the injection. Here are some specific types of microinjection:
- Cytoplasmic Microinjection: This involves injecting substances directly into the cytoplasm of the cell. It’s commonly used for introducing RNA, proteins, or other molecules to study their effects on the cell.
- Nuclear Microinjection: Here, the substances (often DNA) are injected directly into the nucleus of the cell. This method is frequently used in genetic engineering and transgenic animal production.
- Intracytoplasmic Sperm Injection (ICSI): A specialized form of microinjection used in in-vitro fertilization. In ICSI, a single sperm is injected directly into an egg to facilitate fertilization, especially in cases of male infertility.
- Embryonic Microinjection: This involves injecting genetic material into embryos, often at a very early developmental stage. It’s a common method for producing transgenic animals.
- Ooplasmic Microinjection: In this method, substances are injected into the ooplasm of oocytes (immature egg cells). It’s used in various research applications and some assisted reproductive technologies.
- Neuronal Microinjection: Used specifically for introducing substances into neurons. This is valuable in neuroscience research to study neuronal functions, responses, and signaling pathways.
Each type of microinjection has its specific applications, advantages, and challenges. The choice of which type to use depends on the research question, the nature of the substance being injected, and the desired outcome.
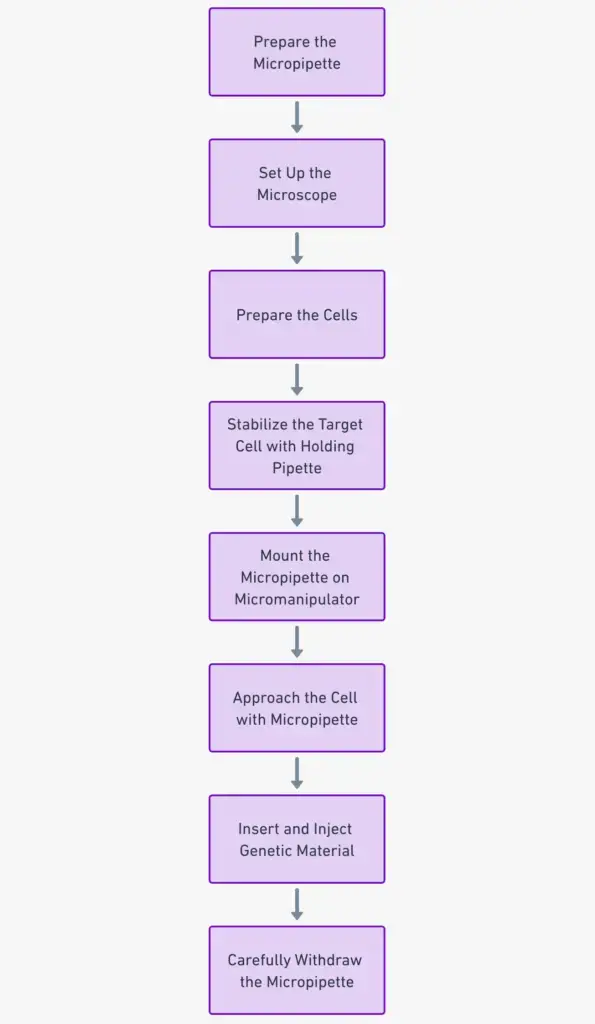
Steps in Microinjection
- Preparing the Micropipette: Start by taking a glass micropipette or needle. Heat one end and stretch it to create a fine tip. This tip, which is about 0.5 mm in diameter, will act like an injection needle.
- Setting Up the Microscope: Since precision is key, the entire microinjection process is carried out under a powerful microscope. This allows for detailed observation and accurate manipulation of the cells.
- Preparing the Cells: Place the cells that you want to microinject into a suitable container.
- Stabilizing the Target Cell: Position a holding pipette close to the cell you want to inject. This pipette uses a gentle suction mechanism to keep the cell steady during the injection.
- Mounting the Micropipette: The micropipette, which holds the material you want to inject, is attached to a device called a micromanipulator. This device ensures that the micropipette can be moved with utmost precision.
- Approaching the Cell: Carefully lower the micropipette towards the cell, ensuring it’s in the right position for injection.
- Insertion and Injection: The micropipette is then gently inserted into the cell, either into its main body (cytoplasm) or its control center (nucleus), based on where you want the genetic material to go. Once in position, a device called a microinjector applies hydrostatic pressure, pushing the genetic material out of the micropipette and into the cell.
- Withdrawal: After the injection is complete, it’s crucial to remove the micropipette from the cell carefully. This step is delicate, as you want to ensure the cell remains intact and undamaged. Using high-quality micropipettes and proper techniques can help reduce the risk of harming the cell.
Alternative Methods of Microinjection
Microinjection is just one of several methods used to introduce genetic material or other substances into cells. Here are some alternative methods to microinjection:
- Transfection: This method involves introducing nucleic acids (like DNA or RNA) into cells using various agents. There are several types of transfection methods:
- Chemical Transfection: Uses cationic lipids or polymers to form complexes with the nucleic acid, facilitating its entry into the cell.
- Calcium Phosphate Transfection: Uses calcium phosphate to precipitate DNA onto cells.
- Electroporation: This method uses an electric field to introduce DNA into cells. The electric field creates temporary pores in the cell membrane, allowing DNA to enter.
- Viral Transduction: Viruses can be engineered to carry specific genes and then used to “infect” target cells, introducing the desired genetic material.
- Agrobacterium-mediated Transformation: Primarily used in plant cells, this method utilizes the bacterium Agrobacterium tumefaciens to transfer genes into plant cells.
- Particle Bombardment (Gene Gun): This method involves coating tiny gold or tungsten particles with DNA and then “shooting” them into cells using a special device.
- Sonoporation: Uses ultrasonic frequencies to create temporary pores in the cell membrane, facilitating the uptake of substances like DNA.
- Magnetofection: Uses magnetic fields to deliver DNA attached to magnetic nanoparticles into target cells.
- Lentiviral Vectors: A type of viral transduction method that can integrate into the host genome, allowing for stable and long-term expression of the introduced gene.
- Heat Shock: In some organisms, especially bacteria, a sudden increase in temperature can make the cell membranes more permeable, allowing DNA to enter.
- DEAE-Dextran Method: Uses the DEAE-Dextran molecule to form complexes with DNA, facilitating its uptake by cells.
Each of these methods has its own advantages, limitations, and specific applications. The choice of method often depends on the type of cell being targeted, the nature of the experiment, and the desired outcome.
Comparison
Let’s compare microinjection with its alternative methods based on various parameters:
1. Methodology:
- Microinjection: Direct introduction of genetic material into cells using a fine glass needle.
- Transfection: Introduction of nucleic acids into cells using various agents.
- Electroporation: Use of an electric field to create temporary pores in the cell membrane for DNA entry.
- Viral Transduction: Use of viruses to introduce specific genes into target cells.
- Agrobacterium-mediated Transformation: Utilizes bacteria to transfer genes into plant cells.
- Particle Bombardment: “Shooting” DNA-coated particles into cells.
- Sonoporation: Use of ultrasonic frequencies to create temporary pores in the cell membrane.
- Magnetofection: Use of magnetic fields to deliver DNA attached to magnetic nanoparticles.
- Lentiviral Vectors: Viral method allowing for stable and long-term gene expression.
- Heat Shock: Sudden temperature increase to make cell membranes more permeable.
- DEAE-Dextran Method: Uses DEAE-Dextran molecules to form complexes with DNA for uptake.
2. Precision:
- Microinjection: High precision in delivering specific amounts of material.
- Transfection, Electroporation, Sonoporation, Magnetofection: Less precise compared to microinjection; amount of uptake can vary between cells.
- Viral Transduction, Lentiviral Vectors: Precision can vary based on viral titer and infection efficiency.
3. Cell Suitability:
- Microinjection: Suitable for many cell types, including hard-to-transfect cells.
- Transfection: Best for cells that are easily transfected; efficiency varies.
- Electroporation: Suitable for many cell types but can be harsh on cells.
- Agrobacterium-mediated: Primarily for plant cells.
- Particle Bombardment: Suitable for both plant and animal cells.
- Viral Methods: Depends on the viral vector’s tropism.
4. Scalability:
- Microinjection: Low scalability; typically used for individual cells or small cell groups.
- Transfection, Electroporation, Viral Methods: Suitable for larger populations of cells; more scalable.
5. Cell Viability:
- Microinjection: High viability if done correctly.
- Electroporation, Sonoporation: Can be harsh and lead to reduced cell viability.
- Transfection: Varies based on method and reagents used.
6. Integration into Genome:
- Microinjection, Transfection: May or may not integrate.
- Viral Transduction, Lentiviral Vectors: Often integrate into the host genome.
7. Labor and Time Intensity:
- Microinjection: Labor-intensive and time-consuming.
- Transfection, Electroporation: Less labor-intensive compared to microinjection.
8. Cost:
- Microinjection: Equipment can be expensive.
- Transfection, Electroporation: Reagents can be costly, but equipment is a one-time investment.
- Viral Methods: Production of viral vectors can be expensive.
| Parameter | Microinjection | Alternative Methods |
|---|---|---|
| Methodology | Direct introduction using a fine glass needle | Varies (e.g., electric field, viruses, magnetic fields) |
| Precision | High precision | Varies; often less precise |
| Cell Suitability | Suitable for many cell types | Depends on method |
| Scalability | Low; individual cells/small groups | Often higher; larger cell populations |
| Cell Viability | High if done correctly | Varies; some methods can be harsh |
| Integration into Genome | May or may not integrate | Depends on method (e.g., viral methods often integrate) |
| Labor & Time Intensity | Labor-intensive & time-consuming | Varies; often less labor-intensive |
| Cost | Expensive equipment | Varies; some methods have costly reagents or equipment |
Advantages of Microinjection
Microinjection, as a method of introducing genetic material into cells, offers several advantages over other gene transfer techniques. Here’s a breakdown of its benefits:
- No Need for Selection Markers: One of the standout features of microinjection is that it doesn’t rely on selection markers, such as antibiotic-resistance genes. This simplifies the procedure by eliminating the need for extra steps to pinpoint and separate transformed cells.
- Precision in Delivery: Microinjection stands out for its accuracy. It allows for the exact delivery of materials, both in terms of the amount and the timing. This level of precision can be hard to achieve with other methods like electroporation or transfection.
- Easy Identification of Injected Cells: To make the process even more efficient, cells that have been microinjected can be quickly identified. This is done by co-injecting a marker dye or proteins that emit fluorescence, making the cells easily distinguishable under a microscope.
- Less Protein Preparation Required: If you’re working with proteins that aren’t abundant or are costly, microinjection is a boon. It demands less protein preparation compared to methods like electroporation. This can save both time and resources.
- Gentler on Cells: One of the primary concerns in any cellular procedure is the well-being of the cells. Microinjection is relatively gentle, causing less stress to cells. As a result, there’s a reduced risk of cell death, which is often seen with other methods like chemical transfection or using viruses to introduce genetic material.
Disadvantages of Microinjection
While microinjection offers several advantages in the realm of molecular biology, it’s essential to be aware of its limitations. Here’s a breakdown of the challenges associated with this method:
- Need for Vehicle Controls: To ensure accurate results and assess any potential effects on cell health, microinjection requires the use of vehicle controls. This adds an extra layer of complexity to the process.
- Technical Expertise: Mastering the microinjection technique demands a high level of skill. Maintaining the health and viability of cells during the process requires specialized training and expertise.
- Popularity of Alternative Techniques: Other methods like transfection, infection, and electroporation have gained traction in recent years. These techniques are often preferred for introducing foreign materials into individual cells, making microinjection less popular for certain applications.
- Time and Labor Intensive: Manual microinjection can be a lengthy process, especially when dealing with numerous cells. This makes it less suitable for experiments that require high-throughput or large-scale applications.
- Limited Scalability: Microinjection typically focuses on a small group of cells within a more extensive culture. This limitation can pose challenges when trying to scale up the process.
- Not Suitable for Large Cell Numbers: If you’re looking to transfer genetic material into a vast number of cells, especially for techniques like Western blotting or purification, microinjection might not be the best choice.
- Challenges with Certain Proteins: Directly microinjecting specific proteins, such as membrane proteins or neurotransmitter receptors, can be tricky and may require alternative methods.
Applications of Microinjection
Microinjection, with its precise delivery mechanism, has found applications in various scientific domains. Let’s delve into some of its primary uses:
- Transgenic Animals Production: Microinjection is a go-to method for producing transgenic animals. By introducing foreign DNA into fertilized eggs, scientists can study gene functions and develop models for various diseases.
- Creation of Chimeric Animals: The first chimeric transgenic mice were produced using microinjection. These chimeric animals, which possess cells from different sources, have been instrumental in understanding embryonic development, cell lineage, and the intricacies of tissue transplantation.
- In Vitro Fertilization (IVF): In the realm of IVF, microinjection plays a pivotal role in a procedure called intracytoplasmic sperm injection (ICSI). This technique involves injecting individual sperm, especially those with movement issues, directly into isolated egg cells. ICSI has been a beacon of hope for many facing male infertility, leading to successful pregnancies and the birth of healthy babies.
- Somatic Cell Nuclear Transfer (SCNT): Microinjection is also employed in SCNT, a technique aimed at creating genetically identical copies of an organism. This is achieved by transferring the nucleus of a somatic cell into an egg cell that has had its nucleus removed.
- Human Embryo Research: Microinjection has been a valuable tool in human embryo studies, allowing for the precise introduction of materials into cells.
- Neuroscience Applications: In the field of neuroscience, microinjection stands out as a reliable method for working with primary cultured human neurons. It facilitates the delivery of proteins, peptides, and cDNA constructs into the cytosol of human neurons, a task that’s challenging with other gene transfer methods.
- Drug Delivery and Testing: Microinjection is often used in pharmacological studies to deliver precise amounts of drugs or other substances directly into specific cells or tissues. This allows researchers to observe the direct effects of these substances on cellular functions.
- Protein Localization Studies: Scientists can use microinjection to introduce fluorescently labeled proteins into cells. This helps in tracking the movement and localization of these proteins within the cell, providing insights into cellular processes and protein functions.
- Studying Cell Signaling: Microinjection can be used to introduce signaling molecules into cells. This helps researchers understand how cells communicate with each other and respond to external stimuli.
- Gene Silencing: Microinjection can be used to introduce small interfering RNAs (siRNAs) into cells. These siRNAs can silence specific genes, allowing researchers to study the function of those genes by observing what happens when they are turned off.
- Studying Cell Division: By microinjecting labeled molecules that bind to specific cellular structures, researchers can observe the process of cell division in real-time, gaining insights into the mechanics and regulation of this crucial process.
- Developmental Biology: Microinjection is used to introduce materials into early-stage embryos. This allows scientists to study how different factors influence embryonic development and understand the roles of specific genes during the early stages of life.
- Functional Genomics: By introducing specific genes or gene fragments into cells, researchers can study the function of those genes, helping to annotate gene functions in genomic databases.
- Studying Membrane Dynamics: Introducing fluorescently labeled lipids or proteins into cells via microinjection can help researchers understand the dynamics of cellular membranes, including processes like endocytosis and exocytosis.
Difference between Electroporation and Microinjection
Electroporation:
- Definition: Electroporation is a physical and direct transformation method that uses an electric field to introduce DNA into host cells.
- Mechanism: It involves the application of a high-voltage electric pulse to a DNA-containing buffer solution, causing the formation of microscopic pores in plasma membranes, allowing DNA to enter the cells.
- Target Cells: Mainly used for protoplasts and plant cells.
- Efficiency: About 40-50% of cells acquire DNA through electroporation, but only half of these transformed cells survive.
- Advantages: The method is relatively simple, does not alter the biological structure or function of cells, and can be applied to various cells.
- Limitations: Only a portion of the cells obtain and retain the DNA, and the survival rate of transformed cells is about 50%.
- Equipment: Does not require any specialized microscope setup.
- Cell Selection: Selection of transformed cells is not very straightforward.
Microinjection:
- Definition: Microinjection is a physical and direct transformation method that introduces DNA into host cells using a micropipette or fine-tipped glass needle.
- Mechanism: DNA is directly injected into the cytoplasm or nucleus of animal cells (like eggs, oocytes, and embryos) or plant protoplasts.
- Target Cells: Primarily used for animal cells, especially in the production of transgenic mice.
- Efficiency: This method is highly reliable and effective.
- Advantages: The technique is direct, and transformed cells can be easily identified using a dye.
- Limitations: The procedure is labor-intensive, time-consuming, and can only treat a few cells at a time.
- Equipment: Requires a specialized and computerized microscope setup.
- Cell Selection: Selection of transformed cells is easier compared to electroporation.
| Parameter | Electroporation | Microinjection |
|---|---|---|
| Definition | Physical method using an electric field to introduce DNA. | Physical method using a micropipette to introduce DNA. |
| Mechanism | High-voltage pulse creates pores in membranes, allowing DNA entry. | Direct injection of DNA into the cytoplasm or nucleus of a cell. |
| Target Cells | Protoplasts and plant cells. | Primarily animal cells (e.g., eggs, oocytes, embryos). |
| Efficiency | 40-50% of cells acquire DNA; about 50% of these transformed cells survive. | Highly reliable and effective. |
| Advantages | Simple and versatile method; can be applied to various cells. | Direct method; transformed cells can be easily identified using a dye. |
| Limitations | Partial DNA acquisition and survival rate; selection of transformed cells is not straightforward. | Labor-intensive, time-consuming, and treats only a few cells at a time. |
| Equipment | Does not require a specialized microscope setup. | Requires a specialized and computerized microscope setup. |
| Cell Selection | Selection of transformed cells is not very straightforward. | Easier selection of transformed cells due to direct method and potential use of identifying dyes. |
FAQ
What is microinjection?
Microinjection is a technique used to introduce genetic material or other substances directly into cells using a fine glass needle or micropipette.
How does microinjection differ from other gene transfer methods?
Unlike some other methods, microinjection allows for the direct and precise delivery of materials into specific parts of a cell, such as the nucleus or cytoplasm.
What are the main applications of microinjection?
Microinjection has applications in creating transgenic animals, studying gene function, in vitro fertilization (specifically ICSI), genetic engineering, and neuroscience research.
Is microinjection suitable for all cell types?
While microinjection can be used for many cell types, its success can vary depending on the cell’s size, structure, and sensitivity.
What are the advantages of microinjection over other methods?
Microinjection offers high precision, doesn’t require selection markers, and is less stressful to cells, reducing cell death compared to some other methods.
Are there any limitations or disadvantages to microinjection?
Yes, microinjection can be labor-intensive, requires technical expertise, and may not be suitable for large-scale or high-throughput experiments.
What is the principle behind microinjection?
The principle of microinjection is based on the direct delivery of genetic material into individual cells using a micropipette, micromanipulator, and a microinjector, typically under a microscope.
How is the success of microinjection measured?
Success can be measured by the survival rate of injected cells, the integration of the introduced material into the cell’s genome, and the desired functional outcomes based on the experiment’s objectives.
What equipment is essential for microinjection?
Key equipment includes a micropipette, micromanipulator, microinjector, and a high-resolution microscope.
Can microinjection be used for therapeutic purposes in humans?
While microinjection is primarily a research tool, it has therapeutic applications, especially in assisted reproductive technologies like intracytoplasmic sperm injection (ICSI) for treating certain types of infertility.
References
- Dean, D. A. (2013). Microinjection. Brenner’s Encyclopedia of Genetics, 409–410. doi:10.1016/b978-0-12-374984-0.00945-1
- Evans TC. Transformation and microinjection. 2006 Apr 6. In: WormBook: The Online Review of C. elegans Biology [Internet]. Pasadena (CA): WormBook; 2005-2018. Available from: https://www.ncbi.nlm.nih.gov/books/NBK19648/
- Jinturkar, K. A., Rathi, M. N., & Misra, A. (2011). Gene Delivery Using Physical Methods. Challenges in Delivery of Therapeutic Genomics and Proteomics, 83–126. doi:10.1016/b978-0-12-384964-9.00003-7
- https://www.news-medical.net/life-sciences/Micromanipulation-and-Microinjection-Techniques.aspx
- https://en.wikipedia.org/wiki/Microinjection
- https://www.slideshare.net/RANASAHA18/microinjection-gene-transfer-method-147612495
- https://link.springer.com/chapter/10.1007/978-3-0348-8705-2_1
- https://www.jove.com/v/54055/microinjection-for-transgenesis-genome-editing-threespine