What is Lysosome?
- Lysosomes are specialized, membrane-bound vesicles present within eukaryotic cells, predominantly in animal cells. These organelles are characterized by their capacity to house hydrolytic enzymes, facilitating the breakdown of a wide array of biomolecules. The term “lysosome” is derived from the Greek words “lysis,” signifying breakdown, and “soma,” denoting body. This nomenclature aptly captures the lysosome’s primary function: the intracellular and, under certain conditions, extracellular degradation of substances.
- Structurally, lysosomes are dense granular entities enveloped by a lipid bilayer membrane. Their internal environment, or lumen, maintains an acidic pH (approximately 4.5–5.0), which is optimal for the hydrolytic enzymes they contain. This acidic milieu is analogous to the stomach’s activity, where enzymes operate at their peak efficiency.
- The genesis of lysosomes is a collaborative effort between the endoplasmic reticulum and the Golgi apparatus. Enzymes destined for lysosomal activity are synthesized in the rough endoplasmic reticulum and subsequently transported to the Golgi apparatus. Here, they are tagged with a specific molecule, mannose 6-phosphate, ensuring their correct sorting into acidified vesicles. These vesicles then fuse with larger acidic vesicles, culminating in the formation of mature lysosomes.
- Lysosomes play a pivotal role in various cellular processes. They are instrumental in digesting materials that the cell internalizes, a process known as endocytosis. Moreover, they recycle intracellular materials, ensuring the cell’s efficient functioning. This recycling process involves the lysosome fusing with a food vacuole, leading to the transfer of hydrolytic enzymes into the vacuole and subsequent digestion of its contents. Furthermore, lysosomes are involved in autophagy, where they digest cellular components, ensuring cellular homeostasis.
- Notably, lysosomes are not uniform in size. Their dimensions can vary significantly, with some being over ten times larger than others. The diversity in their enzymatic content is equally remarkable, with over 60 distinct enzymes identified. These enzymes, along with more than 50 membrane proteins, are regulated by transcription factor EB (TFEB), which modulates the transcription of nuclear genes associated with lysosomal function.
- However, the significance of lysosomes extends beyond their digestive capabilities. Mutations in the genes encoding lysosomal enzymes can lead to lysosomal storage diseases. These disorders arise from the accumulation of specific substrates that the cell cannot degrade. Such genetic anomalies have been linked to various ailments, including neurodegenerative disorders, cancers, cardiovascular diseases, and aging-related conditions.
- In summary, lysosomes are integral cellular organelles responsible for the degradation and digestion of biomolecules. Their presence and activity are paramount for maintaining cellular health and homeostasis. Their intricate relationship with other cellular structures and their role in various diseases underscore their importance in cellular biology and medicine.
Definition of Lysosome
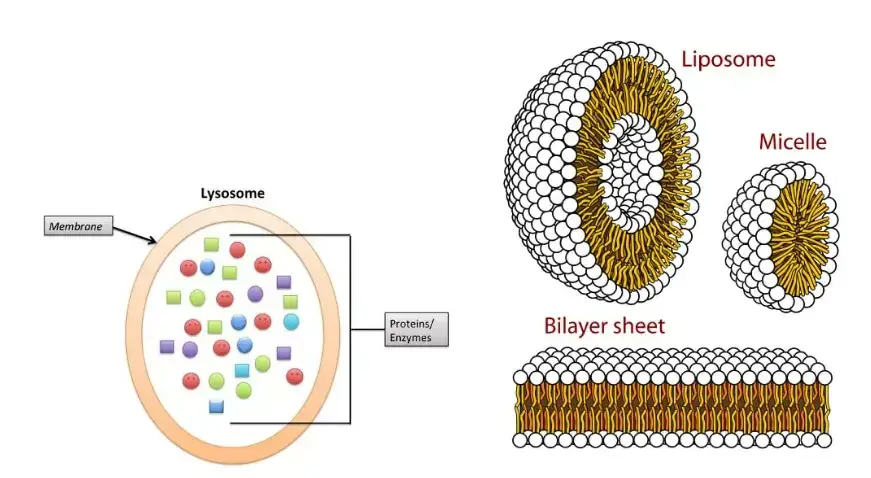
Lysosomes are membrane-bound organelles in animal cells containing hydrolytic enzymes that digest cellular waste, debris, and foreign substances, facilitating intracellular and occasional extracellular degradation.
Characteristics of Lysosomes
Lysosomes are specialized organelles within eukaryotic cells, often described as the cell’s “digestive system.” Their primary function is to degrade and recycle various cellular components, ensuring cellular homeostasis. Herein, we elucidate the key characteristics of lysosomes:
- Morphology and Size: Lysosomes typically manifest as spherical vesicles, with sizes ranging from 0.1 to 1 μm. Their appearance can be homogeneous or heterogeneous, depending on the contents they house.
- Enzymatic Composition: Lysosomes are repositories for approximately 50 hydrolytic enzymes. These enzymes function optimally in acidic environments, a characteristic pH maintained within the lysosomal lumen, which ranges between 4.5 to 5.0.
- Membrane Structure: Each lysosome is encapsulated by a membrane, creating a distinct lumen or cavity. This membrane acts as a protective barrier, ensuring that the potent hydrolytic enzymes within the lysosome do not inadvertently damage other cellular components.
- Origin and Synthesis: The hydrolytic enzymes housed within lysosomes are synthesized in the rough endoplasmic reticulum. Following synthesis, these enzymes undergo post-translational modifications in the Golgi apparatus, where they are tagged with mannose 6-phosphate. Subsequently, they are packaged into vesicles that bud off from the Golgi apparatus, culminating in the formation of lysosomes.
- Functional Diversity: Lysosomes play pivotal roles in various cellular processes:
- Autophagy: Lysosomes degrade worn-out cellular components, a process termed autophagy. Here, cellular debris is enveloped by vesicles, forming autophagosomes, which subsequently fuse with lysosomes for degradation.
- Endocytosis: Lysosomes are involved in the degradation of extracellular substances engulfed by the cell through endocytosis. These substances are enclosed within phagosomes, which fuse with lysosomes to form phagolysosomes, facilitating degradation.
- Exocytosis: Lysosomes can also expel their enzymatic contents outside the cell, aiding in the breakdown of extracellular materials. This is particularly evident in white blood cells, which deploy lysosomal enzymes to degrade foreign invaders.
- Electron Microscopy Appearance: Under electron microscopy, lysosomes are discernible as the most densely stained organelles within the cell’s cytoplasm. Their dark appearance, often darker than mitochondria, makes them easily identifiable.
- Historical Context: Lysosomes were first identified by the Belgian cytologist Christian de Duve in 1949. Their discovery has since illuminated our understanding of cellular maintenance and degradation processes.
- Association with the GERL System: Lysosomes are part of the GERL (Golgi-Endoplasmic Reticulum-Lysosome) system, which is intricately involved in cellular processes like endocytosis and exocytosis.
In essence, lysosomes, with their unique structural attributes and enzymatic arsenal, serve as the cell’s primary degradation and recycling centers, ensuring cellular health and functionality.
Types of Lysosomes
Lysosomes, often termed the “digestive compartments” of eukaryotic cells, are specialized vesicles equipped with hydrolytic enzymes. Depending on their structural attributes and functional roles, lysosomes can be classified into four distinct categories:
- Primary Lysosomes: These are nascent vesicles that emerge from the Golgi apparatus. Characterized by their relatively diminutive size, primary lysosomes house enzymes in an inactive state. Their primary role is to serve as reservoirs of hydrolytic enzymes awaiting activation.
- Secondary Lysosomes: Often referred to as hetero-phagosomes or digestive vacuoles, secondary lysosomes arise from the amalgamation of a primary lysosome with a phagosome, which contains engulfed extracellular material. Within this merged entity, the previously dormant enzymes of the primary lysosome are activated, initiating the degradation of the encapsulated substances.
- Residual Bodies (Tertiary Lysosomes): These are the end products of the lysosomal digestive process. Residual bodies contain remnants of materials that are resistant to lysosomal degradation. These indigestible residues remain sequestered within the cell until they are eventually exocytosed or retained.
- Autophagic Vacuoles (Autophagosomes or Autolysosomes): This category encompasses lysosomes that engage in the process of autophagy. Autophagic vacuoles form when multiple primary lysosomes coalesce around deteriorated intracellular organelles or components. The subsequent degradation of these cellular constituents aids in the recycling of cellular materials and ensures cellular homeostasis. Given their role in internal waste management, lysosomes are aptly dubbed the cell’s “disposal units.”
In essence, the diverse types of lysosomes underscore the organelle’s multifaceted role in cellular maintenance, from digesting extracellular materials to managing intracellular waste.
Lysosome Enzymes
Lysosomes, the cellular organelles responsible for the degradation of both extracellular and intracellular materials, are replete with a specific set of enzymes termed hydrolases. These enzymes are pivotal in facilitating the breakdown of diverse biomolecules within the lysosomal environment.
The repertoire of enzymes within lysosomes is vast, with approximately 40 distinct varieties identified. These enzymes can be broadly categorized based on the substrates they act upon:
- Proteases: These enzymes specialize in the hydrolysis of proteins, breaking down peptide bonds and converting proteins into their constituent amino acids.
- Lipases: Focused on lipids, lipases catalyze the breakdown of fat molecules into fatty acids and glycerol.
- Amylase: This enzyme targets carbohydrates, facilitating their conversion into simpler sugars.
- Nucleases: Nucleases are adept at degrading nucleic acids, cleaving the phosphodiester bonds in DNA and RNA molecules.
- Phosphoric Acid Monoesters: These enzymes are involved in the hydrolysis of phosphoric acid esters.
The overarching characteristic of these enzymes, as hydrolases, is their ability to cleave substrates by incorporating water molecules into the reaction. This hydrolytic mechanism is fundamental to their function. Moreover, the optimal activity of most lysosomal enzymes is observed in an acidic milieu, aligning with the inherently acidic environment of the lysosome.
In summation, lysosomal enzymes, with their diverse range and specificity, play a crucial role in maintaining cellular homeostasis by ensuring the efficient degradation of various biomolecules within the lysosomal compartment.
Structure of Lysosome
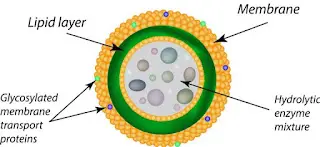
Lysosomes, often referred to as the “digestive compartments” of eukaryotic cells, are specialized organelles equipped with hydrolytic enzymes. Their structural and functional attributes are tailored to facilitate the degradation of various cellular components. Herein, we delve into the intricate architecture of lysosomes:
- Morphology: Lysosomes exhibit pleomorphism, meaning they lack a fixed or characteristic shape. Predominantly, they present as globular or granular entities.
- Size and Membrane: Typically ranging between 0.1 μm to 0.6 μm, with some larger variants extending up to 1.2 μm, lysosomes are encapsulated by a single lipoprotein membrane. This membrane is distinct in its composition and is crucial for maintaining the organelle’s integrity.
- Membrane Proteins: The lysosomal membrane is adorned with specialized proteins, notably lysosomal associated membrane proteins (LAMP) and lysosomal integral membrane proteins (LIMP). These proteins form a protective coat on the membrane’s inner surface, safeguarding it from potential degradation by the hydrolytic enzymes housed within.
- Proton Pump and pH Regulation: A pivotal feature of the lysosomal membrane is the presence of a hydrogen proton pump. This pump actively translocates H+ ions into the lumen, ensuring an acidic environment with a pH ranging between 4.5 and 5.0. This acidic milieu is analogous to the stomach’s acidity and is vital for the optimal activity of lysosomal enzymes.
- Lumen and Enzymatic Content: The internal compartment of the lysosome, termed the lumen, is a repository of hydrolytic enzymes. These enzymes, often found in a crystalline form, are responsible for the degradation of various cellular components, from damaged organelles to foreign entities. The lumen’s content can vary in consistency, from being almost solid to having a differentiated denser periphery and a central granular mass.
- Functional Versatility: Beyond their primary role in degrading biological polymers, lysosomes partake in a plethora of cellular processes. These include discharging cellular materials, energy metabolism, cell signaling, and even participating in the repair mechanisms of the plasma membrane.
- Ubiquity in Eukaryotic Cells: Lysosomes are ubiquitous in animal cells, with the notable exception of red blood corpuscles. Their presence underscores their fundamental role in cellular maintenance and homeostasis.
In summation, lysosomes, with their unique structural attributes and enzymatic arsenal, serve as the cell’s primary degradation and recycling centers, ensuring cellular health and functionality.
Formation of Lysosome
Lysosomes, the cellular “digestive compartments,” are integral to the recycling and degradation of various cellular components. Their formation is a complex, multi-step process intricately linked to the dynamic membrane exchange system of the cell. Here, we elucidate the formation and maturation of lysosomes:
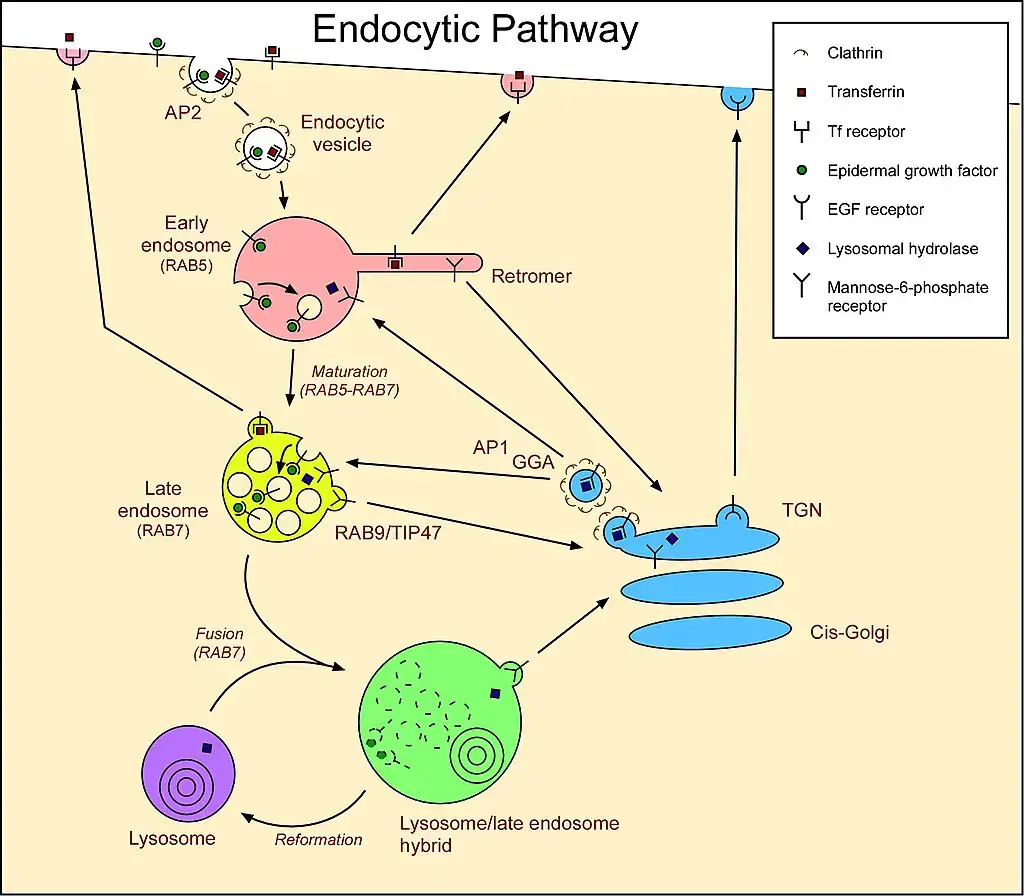
- Endocytosis and Membrane Dynamics:
- Cells continually recycle their components, often by enveloping them within membrane sections. Specifically, during macropinocytosis, a subtype of endocytosis, segments of the plasma membrane pinch off to form vesicles. These vesicles subsequently merge with specific intracellular organelles. Without a replenishment mechanism, the plasma membrane would progressively diminish. Lysosomes are believed to be integral to this membrane exchange, originating from endosomes through a maturation process.
- Lysosomal Protein Synthesis:
- The genesis of lysosomal proteins offers insights into lysosome maintenance. Genes encoding lysosomal proteins undergo transcription in the nucleus, a process orchestrated by the transcription factor EB (TFEB). The resultant mRNA transcripts migrate to the cytosol, where ribosomes translate them. These emerging peptide chains are then translocated into the rough endoplasmic reticulum (ER) for modification. Upon exiting the ER, these proteins, encapsulated in COPII-coated vesicles, are directed to the Golgi apparatus. Here, they receive a specific lysosomal tag, mannose 6-phosphate. This tag facilitates their binding to mannose 6-phosphate receptors in the Golgi, ensuring their correct packaging into vesicles destined for the lysosomal pathway.
- Maturation and Fusion:
- Post-Golgi, vesicles laden with lysosomal enzymes merge with late endosomes, organelles characterized by their mildly acidic environment (pH ~5.5). This acidity prompts the detachment of lysosomal enzymes from the mannose 6-phosphate receptors. Subsequently, these enzymes are compartmentalized into vesicles for transport to pre-existing lysosomes. Over time, the late endosome itself can evolve into a mature lysosome. This maturation is evidenced by the shuttling of endosomal membrane components between lysosomes and endosomes.
In essence, the formation of lysosomes is a testament to the cell’s intricate and dynamic processes, ensuring the efficient recycling and degradation of cellular components.
Why Lysosomes are Known as Suicidal Bags?
- Lysosomes, often described as the cell’s “digestive system,” are equipped with a potent arsenal of hydrolytic enzymes, including proteases, lipases, and nucleases. These enzymes are adept at dismantling a wide array of biological polymers, such as proteins, nucleic acids, carbohydrates, and lipids.
- Their primary function is to degrade and recycle cellular components that are either extraneous or have outlived their utility.
- However, the very strength of lysosomes can also be their vulnerability. In certain circumstances, these enzymes can act upon the lysosomes themselves, leading to their rupture. When this occurs, the potent enzymes are released into the cell’s cytoplasm, where they can indiscriminately digest cellular components.
- This uncontrolled enzymatic activity can lead to the cell’s demise, a process termed autolysis. Derived from the Greek words “auto” (self) and “lysis” (breaking or dissolution), autolysis aptly describes the self-destruction of the cell due to the action of its own lysosomal enzymes.
- Given their potential to instigate cellular self-destruction, lysosomes have earned the moniker “suicidal bags.” While their primary role is to maintain cellular homeostasis by processing and recycling waste, their inadvertent activation can lead to the very destruction of the cell they are meant to protect.
- This dual nature underscores the delicate balance of cellular processes and the pivotal role lysosomes play in cellular health and survival.
Lysosomal Storage Diseases
Lysosomal storage diseases (LSDs) represent a group of inherited metabolic disorders stemming from the malfunctioning of lysosomes. These disorders are characterized by the accumulation of undegraded molecules within cells due to specific enzyme deficiencies. Herein, we delve into the intricacies of LSDs:
- Nature and Prevalence:
- LSDs encompass approximately 50 distinct conditions. Individually, each LSD is rare, with incidences less than 1 in 100,000 births. However, cumulatively, the prevalence of LSDs is estimated to be between 1 in 5,000 to 10,000 births.
- Enzymatic Deficiency:
- The crux of LSDs lies in the deficiency of specific enzymes responsible for breaking down large molecules, such as proteins or lipids. The absence or reduced activity of these enzymes leads to the accumulation of these molecules within cells, culminating in cellular dysfunction and death.
- Inheritance Patterns:
- The majority of LSDs are inherited in an autosomal recessive manner. This entails that an individual must inherit two copies of the mutated gene, one from each parent, to manifest the disease. A classic example of an autosomal recessive LSD is Tay-Sachs disease. In this condition, a deficiency in the enzyme hexosaminidase A results in the accumulation of glycolipids in the brain. This buildup disrupts neural functions, leading to a progressive decline in physical and mental capabilities. Regrettably, affected individuals rarely survive beyond early childhood.
- A subset of LSDs is X-linked, meaning the causative mutation resides on the X chromosome. Fabry disease exemplifies this category. Afflicted individuals lack the enzyme alpha-galactosidase A, leading to the accumulation of the glycolipid globotriaosylceramide. Clinical manifestations range from fatigue, pain, cardiac and renal complications, to distinctive skin lesions termed angiokeratomas. Given its X-linked inheritance, males, possessing a single X chromosome, typically exhibit more pronounced symptoms. Conversely, females, with two X chromosomes, can also manifest symptoms, albeit often milder. The life expectancy for Fabry disease patients varies, with males averaging 58.2 years and females 75.4 years in the United States.
In summation, lysosomal storage diseases underscore the critical role of lysosomes in cellular homeostasis. The absence or malfunction of even a single enzyme can have profound physiological repercussions, emphasizing the intricate balance of cellular processes.
Lysosome in Plant Cell
- Lysosomes, traditionally recognized as the cellular recycling centers in animal cells, have been long understood to be absent in plant cells. Instead, plant cells have been believed to rely on vacuoles to perform the degradative and recycling functions analogous to lysosomes.
- However, recent advancements in cellular biology have prompted a reevaluation of this understanding. While vacuoles in plant cells are multifunctional organelles primarily known for their roles in osmoregulation, storage, and turgor pressure maintenance, they also harbor hydrolytic enzymes. These enzymes are strikingly similar to those found in lysosomes of animal cells, suggesting a shared degradative function.
- This overlap in enzymatic composition has led to a burgeoning debate among botanists and cell biologists. Some argue that the distinction between vacuoles and lysosomes is more semantic than functional, given the similarities in their enzymatic profiles and roles in degradation. In essence, one could posit that the vacuoles in plant cells serve as their version of lysosomes, executing parallel functions in cellular waste management and recycling.
- In conclusion, while traditional cell biology has drawn clear lines between lysosomes and vacuoles based on their presence in animal versus plant cells, emerging evidence suggests a more nuanced understanding. It underscores the idea that, despite differences in nomenclature and cellular architecture, the fundamental processes of cellular maintenance and recycling are conserved across diverse life forms.
Different enzymes present in Lysosomes
Lysosomes, often termed the “digestive compartments” of cells, house a diverse array of enzymes that facilitate the breakdown of various substrates. These enzymes operate optimally in the acidic environment of the lysosome and play pivotal roles in cellular recycling and degradation. Here, we catalog the different enzymes present in lysosomes:
- Phosphatases:
- Acid Phosphatase: Targets most phosphomonoesters.
- Acid Phosphodiesterase: Acts on oligonucleotides and phosphodiesterase.
- Nucleases:
- Acid Ribonuclease: Specifically degrades RNA.
- Acid Deoxyribonuclease: Targets DNA for hydrolysis.
- Polysaccharide/Mucopolysaccharide Hydrolyzing Enzymes:
- β-Galactosidase: Hydrolyzes galactosides.
- α-Glucosidase: Acts on glycogen.
- α-Mannosidase: Targets mannosides and glycoproteins.
- β-Glucoronidase: Degrades polysaccharides and mucopolysaccharides.
- Lysozymes: Hydrolyzes bacterial cell walls and mucopolysaccharides.
- Hyaluronidase: Acts on hyaluronic acids and chondroitin sulfates.
- Arylsulphatase: Targets organic sulfates.
- Proteases:
- Cathepsin(s): Hydrolyzes proteins.
- Collagenase: Specifically degrades collagen.
- Peptidase: Acts on peptides.
- Lipid Degrading Enzymes:
- Esterase: Targets fatty acyl esters.
- Phospholipase: Hydrolyzes phospholipids.
- Sulfatases:
- Arylsulfatase (A, B & G): Acts on O- and N-sulfate esters.
- Glucosamine (N-acetyl)-6-Sulfatase/GNS: Targets glycosaminoglycans.
- Iduronate 2-Sulfatase/IDS: Hydrolyzes O- and N-sulfate esters.
In essence, the diverse enzymatic repertoire of lysosomes underscores their pivotal role in cellular homeostasis. Each enzyme, with its specific substrate affinity, ensures that a wide range of cellular components can be efficiently degraded and recycled.
Where are Lysosomal Enzymes made?
- Lysosomal enzymes are vital components responsible for the degradation and recycling processes within cells. These enzymes are synthesized in the endoplasmic reticulum (ER), a multifunctional organelle that plays a pivotal role in protein synthesis, folding, and post-translational modifications.
- Once synthesized, these enzymes undergo specific modifications, such as glycosylation, which aids in their proper folding and stability. Additionally, they may undergo phosphorylation, which can regulate their activity and targeting.
- Following their synthesis and initial modifications in the ER, these enzymes are then transported to the Golgi apparatus. The Golgi apparatus, another essential cellular organelle, further processes, sorts, and packages these enzymes. It is here that they are directed towards their final destination, the lysosomes.
- Once packaged appropriately, these enzymes are incorporated into vesicles that eventually mature into lysosomes. These lysosomes can then fuse with other vesicles like endosomes or autophagosomes, which contain cellular materials destined for degradation.
- Upon fusion, the lysosomal enzymes come into contact with these materials and begin the process of breaking them down. This degradation is crucial for maintaining cellular homeostasis, ensuring that cells efficiently recycle their components and rid themselves of waste.
- In summary, the synthesis and maturation journey of lysosomal enzymes commence in the endoplasmic reticulum, undergo further processing in the Golgi apparatus, and culminate in their functional deployment within the lysosomes.
Significance of Lysosomes
Lysosomes, often termed the “cellular recycling centers,” play a multifaceted role in ensuring the optimal functioning and survival of cells. Their significance spans across various biological processes, underscoring their indispensable nature. Here, we elucidate the multifarious roles of lysosomes:
- Defense Mechanism in Leucocytes:
- Lysosomes in white blood cells (leucocytes) are frontline defenders against pathogens. They are equipped to digest foreign proteins, bacteria, and viruses, thereby safeguarding the organism from potential infections.
- Autophagy during Starvation:
- In conditions of nutrient scarcity, lysosomes exhibit a remarkable ability to recycle. They digest stored cellular components, such as proteins, fats, and glycogen, ensuring the cell receives the requisite energy to function.
- Metamorphic Transformation in Amphibians:
- The metamorphosis of a tadpole into a frog is a complex process involving significant tissue remodeling. Lysosomes play a pivotal role in digesting embryonic tissues, such as gills and tail, allowing the organism to transition to its adult form.
- Facilitation of Fertilization:
- Lysosomes, specifically those in the acrosome of sperm cells, have a crucial role in reproduction. Their enzymes digest the protective membrane of the ovum, enabling the sperm to penetrate and initiate the process of fertilization.
- Cellular Maintenance and Homeostasis:
- Lysosomes are central to various cellular processes. They act as the cell’s waste disposal system, ensuring timely removal of cellular debris. Furthermore, they facilitate the recycling of cellular components, ensuring the cell’s longevity and efficient functioning. Additionally, lysosomes are involved in programmed cell death, a process vital for tissue development and maintenance.
In summary, lysosomes, with their diverse enzymatic arsenal, are indispensable for cellular health and function. Their roles, ranging from defense to reproduction, highlight their significance in the intricate tapestry of life.
Functions of Lysosome
Lysosomes, often termed the “digestive compartments” of eukaryotic cells, play pivotal roles in various cellular processes. Their primary function revolves around the degradation and recycling of cellular components. Herein, we elucidate the multifaceted functions of lysosomes:
- Intracellular Digestion:
- Lysosomes are adept at digesting food particles within the cell. Upon the cell’s uptake of food, the lysosomal membrane fuses with the food vacuole’s membrane, releasing its hydrolytic enzymes into the vacuole.
- Subsequent to digestion, the processed nutrients permeate the vacuole membrane, becoming available for cellular energy production and growth.
- Autolytic Action:
- Cells often need to dispose of worn-out or malfunctioning organelles. Through a process termed autophagy, these organelles are enveloped by vesicles or vacuoles, forming an autophagosome.
- The autophagosome subsequently merges with a lysosome, where the entrapped organelles are degraded by lysosomal enzymes.
- Heterophagy:
- This process involves the cellular uptake of external materials via phagocytosis or pinocytosis. Once internalized, the engulfed material is digested following the fusion of the resultant vacuole with a lysosome.
- Autophagy:
- Beyond the degradation of malfunctioning organelles, autophagy is a physiological process essential for cellular homeostasis. It facilitates protein degradation and the turnover of cellular components, paving the way for new cell formation.
- Extracellular Digestion:
- In certain instances, primary lysosomes exocytose their hydrolytic enzymes into the extracellular milieu. This results in the degradation of extracellular substances, a phenomenon observed in organisms like saprophytic fungi.
- Autolysis:
- Autolysis refers to the self-destruction of cells mediated by lysosomal enzymes. This occurs when the lysosomal membrane ruptures, releasing its enzymes into the cytosol. Notably, autolysis plays a role during specific developmental stages, such as amphibian and insect metamorphosis.
- Fertilization:
- During fertilization, the acrosome, a specialized lysosome at the sperm head, ruptures, discharging its enzymes onto the egg’s surface. These enzymes facilitate sperm entry by digesting the egg’s protective membrane.
- Cellular Janitors:
- Lysosomes act as the cell’s custodians, clearing out cellular debris and potential ‘junk’. By doing so, they play a preventative role, warding off potential diseases that might arise from the accumulation of cellular waste.
In summation, lysosomes, with their diverse functions, underscore their indispensable role in cellular maintenance, degradation, and recycling, ensuring cellular health and vitality.
Observation of lysosome under Microscope
Lysosomes can be too small to observe using an optical microscope. This is why electron microscopes are employed to examine the lysosomes. It is nevertheless possible to see the in-cell lysosome (vacuole) within the plant cell. Here is the procedure to examine the vacuole of a plant:
Requirements
- An onion
- Glycerin
- Safraning solution
- A pair of forceps
- A dropper
- Microscope glass slides
- A light compound microscope
- Distilled water
- Microscope cover slip
- Two watch glasses
Procedure
- Drop a few drops pure water into a glass watch
- Utilizing a pair of forceps, cut off the membrane of the skin of an onion. Place it in the glass with the help of a small amount of water.
- Include a few drops safranin into the other watch glass empty.
- Choose the onion’s membrane with the forceps, then place it in the glass with safranin . Allow it to rest for around 30 seconds.
- Take the membrane back and place it back into the watch glass using the distilled water.
- Place a drop of glycerin into the center of a microscope slide
- Put the membrane of onion onto the slide of glass (in the form of glycerin) and cover it with an cover slip
- Slide the slide under the microscope and watch
Observation
In addition to seeing many irregular cells, and the cell nucleus Students will be able to be able to clearly see a huge vacuole that is located in the center inside the cell.
Quiz
Which organelle is often referred to as the “suicide bag” of the cell?
a) Mitochondria
b) Golgi apparatus
c) Lysosome
d) Endoplasmic reticulum
[expand title=”Show answer” swaptitle=”Hide answer”] c) Lysosome [/expand]
Lysosomes are rich in which type of enzymes?
a) Anabolic
b) Catabolic
c) Hydrolytic
d) Oxidative
[expand title=”Show answer” swaptitle=”Hide answer”] c) Hydrolytic [/expand]
Which cellular process involves lysosomes digesting damaged organelles?
a) Endocytosis
b) Exocytosis
c) Autophagy
d) Phagocytosis
[expand title=”Show answer” swaptitle=”Hide answer”] c) Autophagy [/expand]
The internal environment of a lysosome is:
a) Alkaline
b) Neutral
c) Acidic
d) Basic
[expand title=”Show answer” swaptitle=”Hide answer”] c) Acidic [/expand]
Lysosomes originate from which cellular organelle?
a) Nucleus
b) Mitochondria
c) Golgi apparatus
d) Ribosome
[expand title=”Show answer” swaptitle=”Hide answer”] c) Golgi apparatus [/expand]
Which disease is associated with a deficiency in lysosomal enzymes?
a) Tay-Sachs disease
b) Alzheimer’s disease
c) Parkinson’s disease
d) Diabetes
[expand title=”Show answer” swaptitle=”Hide answer”] a) Tay-Sachs disease [/expand]
In plant cells, which organelle performs functions similar to lysosomes?
a) Chloroplast
b) Vacuole
c) Peroxisome
d) Plasmodesmata
[expand title=”Show answer” swaptitle=”Hide answer”] b) Vacuole [/expand]
Which of the following is NOT a function of lysosomes?
a) Protein synthesis
b) Digestion of cellular waste
c) Breakdown of foreign substances
d) Recycling of cellular components
[expand title=”Show answer” swaptitle=”Hide answer”] a) Protein synthesis [/expand]
The enzymes within lysosomes are most active at which pH level?
a) 2-3
b) 4.5-5.0
c) 6-7
d) 8-9
[expand title=”Show answer” swaptitle=”Hide answer”] b) 4.5-5.0 [/expand]
Which process involves the fusion of a lysosome with a vesicle containing foreign material?
a) Autophagy
b) Endocytosis
c) Phagocytosis
d) Exocytosis
[expand title=”Show answer” swaptitle=”Hide answer”] c) Phagocytosis [/expand]
FAQ
What is lysosomes and its function?
Lysosomes are membrane-bound organelles found in animal cells. They contain hydrolytic enzymes that can break down virtually all kinds of biomolecules, including proteins, nucleic acids, carbohydrates, and lipids. Their primary function is to aid the cell in digesting unwanted materials.
What are the three main functions of lysosomes?
The three main functions of lysosomes are:
a) Digesting cellular waste and debris.
b) Breaking down foreign substances that enter the cell.
c) Recycling of cellular components through a process called autophagy.
What is lysosome found in plants or animals?
Lysosomes are predominantly found in animal cells. In plant cells, the functions similar to lysosomes are carried out by vacuoles.
What are lysosomes also called?
Lysosomes are also called “suicidal bags” of the cell due to their ability to cause autolysis if they burst.
What are 5 functions for lysosome?
a) Digesting cellular waste.
b) Breaking down foreign substances.
c) Recycling of cellular components (autophagy).
d) Engaging in programmed cell death (apoptosis).
e) Digesting cellular organelles that are no longer functional.
What are the 4 types of lysosomes?
The four types of lysosomes are:
a) Primary Lysosomes
b) Secondary Lysosomes
c) Tertiary Lysosomes (or Residual Bodies)
d) Autophagic Vacuoles (or Autophagosomes).
Who discovered lysosomes?
Lysosomes were discovered by the Belgian cytologist Christian de Duve in 1949.
Where are lysosomes located?
Lysosomes are located in the cytoplasm of animal cells.
Where are lysosomes made?
Lysosomes are made in the Golgi apparatus from where they bud off as vesicles containing hydrolytic enzymes.
What is the size of the lysosomes?
The size of lysosomes can vary, but they typically range from 0.1 μm to 1.2 μm in diameter.
What are the types of lysosomes?
As mentioned earlier, the types of lysosomes are Primary, Secondary, Tertiary (Residual Bodies), and Autophagic Vacuoles.
How many lysosomes are in a cell?
The number of lysosomes in a cell can vary based on the cell type and its specific functions. Some cells may have just a few, while others might have hundreds.
What is the structure of lysosomes?
Lysosomes are spherical vesicles enclosed by a single lipid bilayer membrane. Inside, they contain a variety of hydrolytic enzymes that function best in an acidic environment.
Why lysosomes is called stomach?
Lysosomes are often referred to as the “stomach” of the cell because they contain digestive enzymes and are responsible for digesting cellular waste and foreign substances, much like how the stomach digests food.
Why are lysosomes called?
Lysosomes are called “lysosomes” because of their lytic (breaking down) properties and their similarity in function to the somatic cells. The term “lysosome” is derived from the Greek words “lysis” (meaning dissolution or destruction) and “soma” (meaning body).
References
- Cooper GM. The Cell: A Molecular Approach. 2nd edition. Sunderland (MA): Sinauer Associates; 2000. Lysosomes. Available from: https://www.ncbi.nlm.nih.gov/books/NBK9953/
- https://www.thoughtco.com/lysosomes-cell-organelles-373357
- https://teachmephysiology.com/histology/cell-structures/lysosomes/
- http://www.biology4kids.com/files/cell_lysosome.html
- https://biologydictionary.net/lysosome/
- https://sciencing.com/lysosome-definition-structure-function-13717289.html
- https://www.microscopemaster.com/lysosomes.html
- https://www.ybstudy.com/2020/07/lysosomes-structure-functions.html
- https://www.biologybrain.com/diagram-of-lysosomes-structure-types/