Lactophenol Cotton Blue (LPCB) staining is the simple wet-mount method used for microscopic examination of fungi, and it is considered one of the common procedure in mycology.
It is the process where fixation, staining, and preservation of fungal structures is achieved within a single reagent. The solution is prepared by phenol, lactic acid, glycerol and cotton blue dye. Phenol kills the fungal organism and also acts as a fixative which prevents autolysis of the fungal elements. Lactic acid acts as a clearing agent and it helps in maintaining the fungal cell wall so that the structures can be seen clearly.
Glycerol is a viscous mounting medium that prevents drying of the specimen on slide. Cotton blue is an aniline dye that stains the chitin of fungal cell wall, and the fungal structures is stained in blue colour. It is the process used mostly for identification of hyphae, spores and other fungal parts under the microscope.
The preparation is stable for some time but it is always performed with care because phenol is corrosive and toxic, and handling is done with proper protective measures.
Principle of Lactophenol Cotton Blue (LPCB) Staining
The principle of Lactophenol Cotton Blue (LPCB) staining is based on the ability of the reagent to fix, preserve and stain the fungal cell wall in a single preparation. It is the process where the components of the solution act together to make the fungal structures visible under the microscope. The fungal cell wall contains chitin, and the cotton blue dye has strong affinity towards this chitin, so the fungal spores and hyphae is stained in blue colour. Phenol present in the solution kills the fungal organism and acts as a fixative which prevent degradation of the fungal structures. Lactic acid helps in clearing and also preserve the morphology of the fungal elements.
Glycerol acts as the mounting medium which prevents drying of the preparation. Cotton blue stains the chitin and gives contrast to the fungal structures. It is the combined action of fixation, clearing, staining and mounting that allow clear observation of the fungal elements in LPCB staining.
Aim of LPCB staining
Microscopic examination of fungal cells by using Lactophenol cotton blue.
Requirements
- Distilled water 50 ml
- Cotton Blue (Aniline Blue) 0.125 g
- Phenol crystals (C6H5O4) 50 g
- Glycerol 100 ml
- Lactic acid (CH3CHOHCOOH) 50 ml
- 70% ethanol
- The solution is prepared at least 2 days before use.
Preparation of Lactophenol Cotton Blue solution
- It is prepared by mixing phenol, lactic acid, glycerol and Cotton blue dye in required proportion.
- In this method the phenol crystals is dissolved first in distilled water and the lactic acid is added slowly with gentle mixing.
- Glycerol is added after this step and the solution is mixed until it becomes uniform without heating.
- Cotton blue (Aniline blue) is then added and it is stirred until the dye is completely dissolved in the lactophenol base.
- Because phenol is highly toxic, the preparation is carried out under a fume hood using protective gloves and eye protection.
- In the two-day procedure, the cotton blue is dissolved in distilled water on Day 1 and kept overnight so that insoluble dye settles at the bottom.
- On Day 2 the phenol crystals is added to lactic acid and stirred using a magnetic stirrer until the crystals dissolve, then glycerol is added.
- The cotton blue solution is filtered and added into the phenol + lactic acid + glycerol mixture and mixed properly.
- It is kept undisturbed for the maturation of the reagent.
- The final lactophenol cotton blue solution is stored in amber bottles to protect it from light and it is kept at 2–30°C.
Procedure of Lactophenol cotton blue Staining
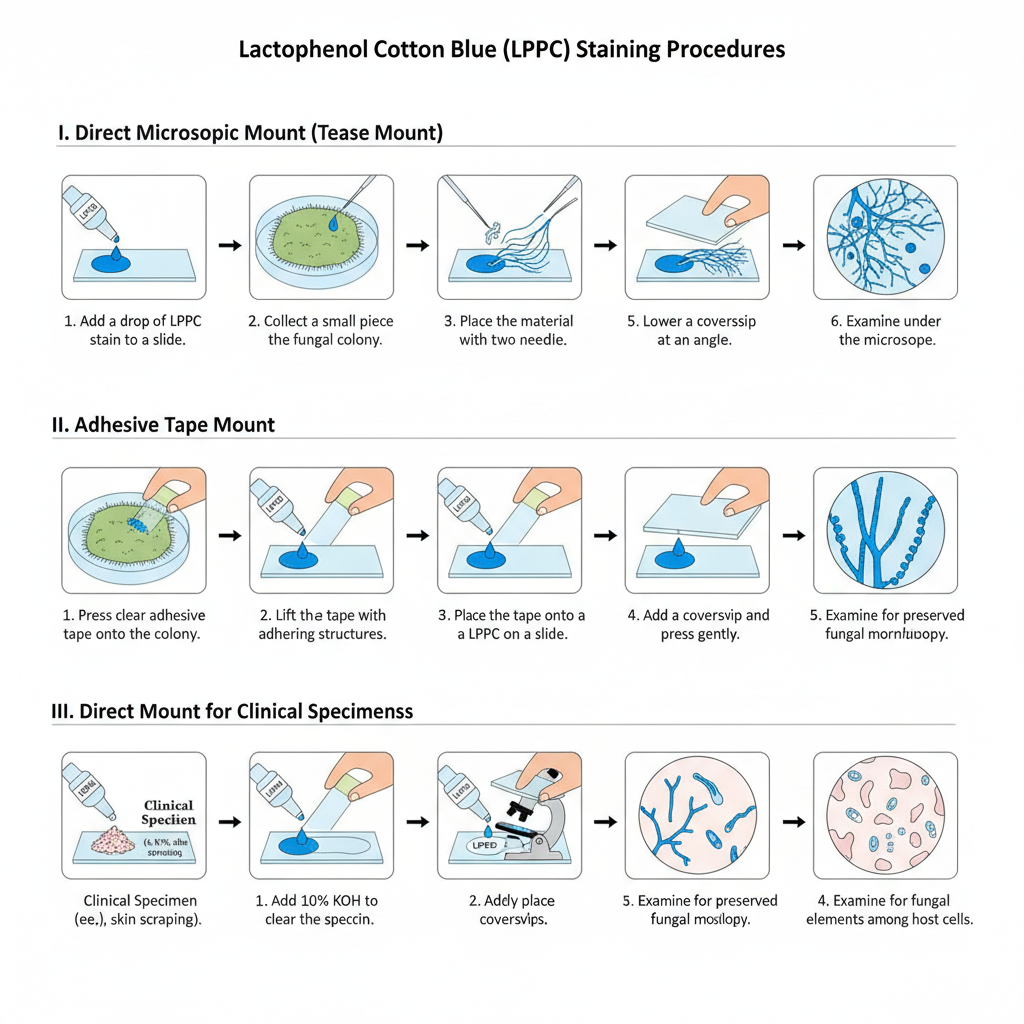
I. Direct Microscopic Mount (Tease Mount)
This is the basic method used for fungal culture.
- A drop of Lactophenol cotton blue stain is placed at the center of a clean slide.
- A small part of the fungal growth (2–3 mm from colony edge) is taken using an inoculating needle.
- This fragment is placed directly in the drop of LPCB stain.
- With the help of two sterile needles the material is teased so that the hyphae is spread thinly in the stain.
- A coverslip is placed gently touching one edge first and lowered slowly to avoid air bubbles.
- The slide is examined under low power (10X or 100X) using reduced light.
- It is then observed under high power (40X or 400X) to study the fungal structure.
- The coverslip edges can be sealed with nail polish for making a permanent mount.
II. Adhesive Tape Mount
This technique is used for quick examination of colony and it preserves the fungal morphology.
- One or two drops of LPCB stain is placed on a clean slide.
- A piece of transparent adhesive tape is taken with sticky side facing outward.
- The sticky surface is pressed gently on the fungal colony to pick the fungal structures.
- The tape is lifted slowly from the colony surface.
- The tape is placed on the LPCB stain either sticky side down or sticky side up.
- If sticky side is up, another drop of LPCB is added on the exposed fungal material.
- A coverslip is placed and pressed gently to remove trapped air.
- The slide is examined initially at 100X and then at 400X.
III. Direct Mount for Clinical Specimens
This is used for skin scrapings, corneal scraping and tissue samples.
- For clearing, two drops of 10% KOH is mixed with the specimen on a clean slide.
- For corneal sample a drop of 70% ethanol is used first to remove lipids and debris.
- One or two drops of LPCB stain is added before the alcohol dries.
- A coverslip is placed slowly making a thin mount without air bubbles.
- The slide is examined first under low power and then under high power for identifying fungal elements.
Result of Lactophenol Cotton Blue Staining
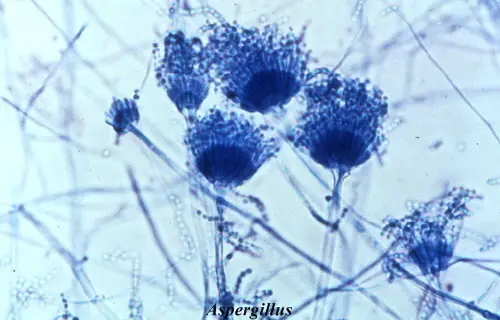
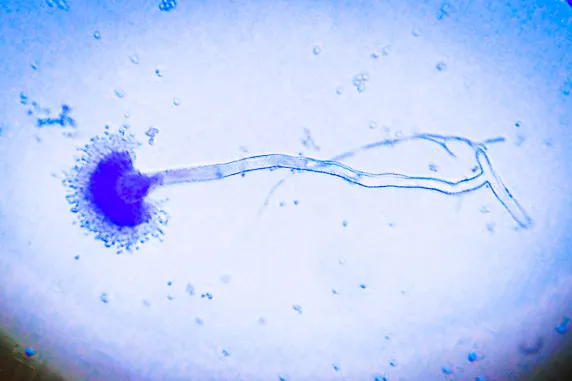
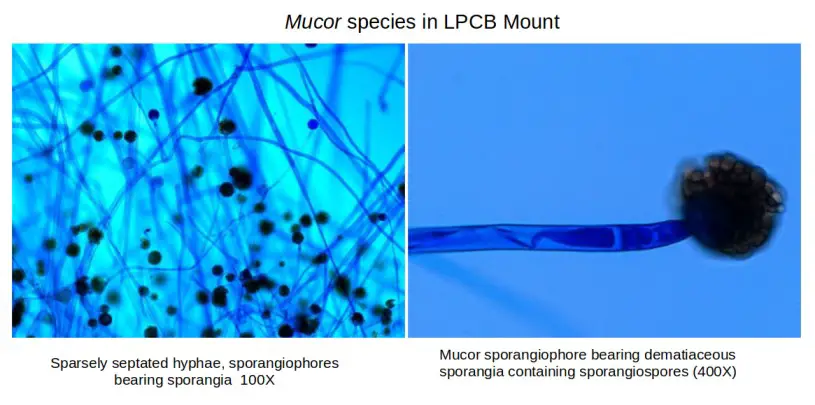
- In this staining the fungal structures is seen clearly because the cotton blue binds with the chitin of the fungal wall.
- The hyphae, spores and fruiting bodies stain deep blue or delicate blue while the background appears pale blue.
- It is the process where the important fungal characters like septation of hyphae, the size and arrangement of conidia and the appearance of mycelial filaments is observed easily.
- Aspergillus niger shows blue coloured hyphae and fruiting structure with pale blue background.
- Trichophyton mentagrophytes shows septate hyphae and the microconidia and macroconidia which stain delicate blue in LPCB mount.
- Some parasites also stain blue, for example the oocysts of Cyclospora and Isospora stain blue in stool mount.
- The eggs of Enterobius vermicularis also take blue colour which helps in identification because the eggs are normally transparent.
- Cryptosporidium is not stained properly in this method.
- This method gives presumptive identification only because some delicate spores may be broken during teasing and further tests is needed for confirmation.
Limitation of Lactophenol Cotton Blue Staining
- The major limitation is the presence of phenol because phenol is highly toxic and corrosive.
- It can cause severe burn on skin and it is dangerous to eye causing permanent injury or blindness.
- The reagent must be handled under fume hood with proper protective gloves and eye protection due to its toxicity.
- Safer alternatives like iodine–glycerol is needed in some routine work because these have less hazard.
- In tease mount method the delicate conidia and spores is often broken and this affects the identification of the fungus.
- Tape mount is temporary only because the lactic acid and glycerol slowly dissolve the adhesive of the tape.
- The stained slides must be examined quickly as long standing storage may change the appearance of fungal elements.
- Direct specimen from patient sometimes need pretreatment with alcohol to remove lipids and debris for clear observation.
- LPCB gives only presumptive identification because it is based mainly on morphological characters.
- It is not useful for identification of Cryptosporidium as this parasite does not stain properly.
- The stain works best on mature fungal colony and the young vegetative forms is not identified clearly.
- Proper interpretation of LPCB mount requires trained personnel.
- It is mainly used for molds and has limited value in yeast identification.
Application of Lactophenol Cotton Blue Staining
- It is mainly used for rapid microscopic examination of fungi and molds.
- The stain helps in observing the organization of hyphae and the shape and arrangement of spores which is important for fungal identification.
- Cotton blue binds with chitin and cellulose of the fungal wall giving a clear blue colour that improves the contrast of the structures.
- It is used for presumptive identification of fungal genera from culture plates and direct mounts.
- It is applied in tease mount and tape mount preparations for studying filamentous fungi like Aspergillus, Penicillium, Fusarium, Trichophyton and Microsporum.
- In mycotic keratitis the corneal scraping is stained with LPCB for quick diagnosis after clearing with 70% alcohol.
- It is also used for examining pure cultures of endophytic fungi and for identification of fungi showing high enzyme activity.
- LPCB is used in stool examination for detecting coccidian parasites like Cyclospora and Isospora where the oocysts stain blue.
- It is used with tape method to detect Enterobius vermicularis eggs which become deep blue making the identification easy.
- Other parasites like trophozoites, cysts and helminth eggs can also be seen in LPCB mount though it is not useful for Cryptosporidium.
- The phenol present in the reagent kills the organism and prevents autolysis thus fixing the structures.
- Lactic acid acts as a clearing agent and preserves the fungal elements by reducing background disturbance.
- Glycerol works as a mounting medium preventing drying and stabilizing the slide for long observation.
Quality Control
- Appearance: The color of Lactophenol cotton blue solution should be Ink blue.
- Visibility: It should be Clear, without any insoluble particles.
- Microscopic Observation: After staining the fungal cell, Fungal Spores and hyphae are observed under microscope using high power (40X) objective lens after staining with Lactophenol cotton blue.
- Results: Fungal spores and hyphae will appear as pale to dark blue.
FAQ
What is Lactophenol Cotton Blue (LPCB) staining?
Lactophenol Cotton Blue (LPCB) staining is a histological staining method used to visualize the morphology and structure of fungal cells.
Why is LPCB staining used?
LPCB staining is used in the study of fungal biology, including the taxonomy and classification of fungi, the study of fungal diseases and infections, and the study of fungal biochemistry and metabolism.
How does LPCB staining work?
LPCB staining works by suspending the fungal cells in a mixture of lactophenol and cotton blue, which penetrates the cell wall and binds to the cell contents. The stained cells are then visualized under a microscope, allowing for the study of the fungal morphology and structure.
What are the components of the LPCB staining solution?
The components of the LPCB staining solution include lactophenol and cotton blue.
Is LPCB staining reliable?
LPCB staining is a widely used and reliable staining method in the study of fungi, with a long history of successful use.
How long does LPCB staining take?
LPCB staining typically takes about 30 minutes to an hour to complete, depending on the specific protocol being used.
Can LPCB staining be performed on fixed or unfixed samples?
LPCB staining is typically performed on unfixed samples, such as fungal cultures, to maintain the integrity of the cells.
What are the limitations of LPCB staining?
The limitations of LPCB staining include the need for specialized equipment, such as a microscope, to visualize the stained cells, as well as the potential for staining artifacts and errors in interpretation.
How are the results of LPCB staining interpreted?
The results of LPCB staining are typically interpreted by a trained mycologist, who will analyze the stained cells under a microscope and determine the type and structure of the fungal cells, as well as any potential abnormalities or disease markers.
Can LPCB staining be used in combination with other staining techniques?
Yes, LPCB staining can be used in combination with other staining techniques, such as Calcofluor White staining, to enhance the resolution and specificity of the results.
- https://en.wikipedia.org/wiki/Methyl_blue
- https://en.wikipedia.org/wiki/Lactophenol_cotton_blue
- https://mycology.adelaide.edu.au/laboratory/lacto/
- https://laboratoryinfo.com/lactophenol-cotton-blue-lpcb/
- https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma-Aldrich/Datasheet/1/61335dat.pdf
- http://www.generalmicroscience.com/microbial-laboratory-techniques/staining-fungus-using-lactophenol-cotton-blue/
- https://www.dalynn.com/dyn/ck_assets/files/tech/SL18.pdf
- https://catalog.hardydiagnostics.com/cp_prod/Content/hugo/LactophenolCottonBlStn.htm
- http://www.himedialabs.com/TD/S016.pdf
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1706009/
- https://microbenotes.com/lactophenol-cotton-blue-staining/
- http://www.biologycourses.co.uk/biomedical-science/biomedical-science-technique-lactophenol-cotton-blue-lpcb
- https://www.dalynn.com/dyn/ck_assets/files/tech/SL18.pdf
very good piece of information … thank you for this informative article… really appreciating…
Very good & informative