The lactophenol cotton blue (LPCB) wet mount preparation is the most used method for staining and viewing fungus, and its preparation is straightforward. The formulation contains the following ingredients:
- Phenol destroys fungus.
- As a cleaning agent, lactic acid aids in the preservation of fungal structures.
- Cotton blue is an aniline dye that stains the chitin in fungal cell walls, hence adding colour to the fungal preparation and intensifying and contrasting the structures.
- The sticky ingredient glycerol prevents the prepared slide specimen from drying out.
As a mounting medium and staining agent, lactophenol cotton blue solution is used to prepare slides for microscopic study of fungus. Elements of fungi are dyed vividly blue. This procedure must always be performed beneath a biological safety hood and with gloves on at all times.
Composition of LPCB mount
| Ingredients | Amount |
| Lactic acid | 20 mL |
| Glycerol (or glycerine) | 40 mL |
| Phenol crystals or phenol concentrate | 20 g 20 mL |
| Distilled water | 20 mL |
| Aniline blue or 1% aqueous solution (This is analogous to cotton blue.) | 0.05 g 2 mL |
Preparation of LPCB mount
LPCB can be acquired from commercial vendors or made at home by combining the aforementioned materials as described below.
- Incorporate 20 mL of lactic acid into a beaker.
- Add 40 mL of glycerol or glycerine.
- Add 20 mL of ultrapure water
- Add 22 grammes of crystallised phenol (22 ml of melted phenol).
- Add 0.05 g. aniline blue.
- To dissolve the stain, heat the fluid and stir it thoroughly. Never boil or exceed 100°C.
- Mix thoroughly and chill the solution. The solution can be stored at room temperature and dispensed using a pipette as needed.
Procedure of Lactophenol Cotton Blue (LPCB) Mounts
Standard Tease Mount
- A drop of 70% alcohol should be placed on a clean microscope slide.
- Material should be extracted from cultures of filamentous fungi using a stiff inoculating wire, not the loop employed for manipulations with bacteria or yeasts.
- Hold the wire erect in the hottest portion of the Bunsen flame, just above the blue cone, until the entire length of the wire emits a reddish-orange glow.
- Before placing the inoculating wire in a fungal culture, it must have cooled sufficiently; after approximately ten seconds, it should have cooled sufficiently.
- Take off the top of the culture tube (but do not put it on the bench). Burn the tube’s neck to eliminate any contaminated bacteria.
- Take out a small portion of the culture.
- It is generally advantageous to take a small amount of the agar media along with the fungus. In any event, the material should be put to the slide with as little disturbance as possible.
- Replace the cap after igniting the tube’s neck a second time with flames.
- Immerse the fungal material in the alcohol droplet.
- This forces the air between the hyphae to escape.
- Using mounted needles*, delicately separate the fabric.
- Don’t forget to sanitise the inoculating wire and needles by heating them to red heat in a Bunsen flame after each use.
- Before the alcohol evaporates, add one to two drops of the stain.
- A common error is adding too much stain to the mixture.
- Touch one edge of the drop of stain with the edge of the coverslip, while holding it between your index finger and your thumb.
- Gently lower the coverslip onto the slide while attempting to avoid air bubbles.
- Your preparation is currently exam-ready.
Teasing the colony frequently damages the delicate fruiting structures of the filamentous fungi, making it impossible to identify the essential spore configurations or hyphal attachments for definite identification. In such instances, a tape mount or microslide culture may be necessary.
Cellophane Tape Preparation
The cellophane or transparent tape approach for the microscopic study of fungi is straightforward, economical, and quick. It enables for a precise identification by preserving the conidial arrangements of the more delicate filamentous moulds.
- Place a drop of aniline blue lactophenol stain on a microscope slide.
- Gently but firmly press the adhesive side of unfrosted, clear cellophane tape to the surface of the colony, capturing a part of the aerial mycelium.
- Attach one end of the tape to the slide surface adjacent to the stain droplet.
- Stretch the tape over the stain and lower it slowly so that the mycelium absorbs the stain.
- Pull the tape taut and adhere the opposing end to the glass, avoiding air bubbles as much as possible.
- Your preparation is currently exam-ready.
Examination and Result
- Perform the initial examination using a low-magnification objective lens. The best photos will result from the thinner portions of the preparation, which are typically located around the margins of the mounting material.
- For a more precise analysis of spores and other structures, switch to a 40X objective with a higher magnification.
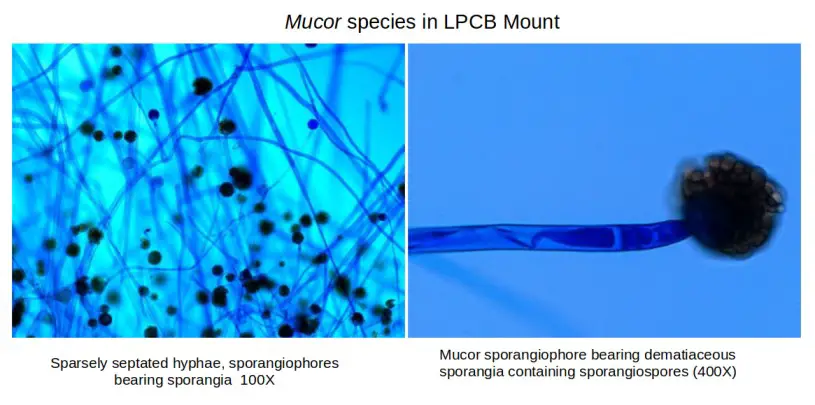
Applications of Lactophenol Cotton Blue (LPCB) Mounts
Lactophenol Cotton Blue (LPCB) mounts are used in the study of fungi to preserve and visualize fungal cultures. LPCB mounts are made by mixing a fungal culture with lactophenol and cotton blue, which penetrates the cell wall and binds to the cell contents. The resulting stained fungal cells are then placed on a microscope slide and covered with a coverslip, creating a permanent mount for observation and study.
LPCB mounts are used in a variety of applications in the study of fungal biology, including:
- Taxonomy and classification of fungi: LPCB mounts allow for the visual examination of fungal morphology, which is important in the classification and identification of different types of fungi.
- Study of fungal diseases and infections: LPCB mounts are used to study the morphology and structure of fungal pathogens, which can provide valuable information on the nature and spread of fungal infections.
- Study of fungal biochemistry and metabolism: LPCB mounts can be used to study the cellular structure and organization of fungal cells, which is important in the understanding of fungal biochemistry and metabolism.
- Quality control in the pharmaceutical industry: LPCB mounts are used to monitor the quality and purity of fungal cultures used in the production of antibiotics and other pharmaceutical products.
- Environmental monitoring: LPCB mounts are used in the study of fungal populations and communities in different environmental settings, such as soil, water, and air.
Overall, LPCB mounts are an important tool in the study of fungal biology, providing a permanent and easily accessible resource for observation and analysis.
Advantages of Lactophenol Cotton Blue (LPCB) Mounts
- Permanent preservation: LPCB mounts provide a permanent preservation of the fungal cells, which can be stored for an extended period of time for future reference and study.
- Easy visualization: The use of lactophenol and cotton blue in the LPCB mount provides clear and easily visible staining of the fungal cells, making them easy to observe and study under a microscope.
- Wide application: LPCB mounts have a wide range of applications in the study of fungal biology, including taxonomy and classification, the study of fungal diseases, and the study of fungal biochemistry and metabolism.
- High contrast: LPCB mounts provide high contrast between the fungal cells and the background, allowing for clear visualization of the cellular structure and organization.
- Cost-effective: LPCB mounts are a relatively cost-effective method of preserving and visualizing fungal cultures, making them accessible to a wide range of researchers and institutions.
Disadvantages of Lactophenol Cotton Blue (LPCB) Mounts
- Specialized equipment required: Observing LPCB mounts requires a microscope, which can be expensive and may not be accessible to all researchers or institutions.
- Staining artifacts: There is a potential for staining artifacts and errors in interpretation when using LPCB mounts, which can affect the accuracy of the results.
- Limited information: LPCB mounts provide limited information on the metabolic and biochemical processes of the fungal cells, as they are primarily used for visualizing cellular structure and organization.
- Potential for sample degradation: Over time, the fungal cells in the LPCB mount may degrade, affecting the quality of the preserved sample and the accuracy of the results.
Overall, while LPCB mounts have several advantages, they also have some limitations and potential disadvantages, which should be considered when using this staining technique.
FAQ
What is Lactophenol Cotton Blue (LPCB) mount?
LPCB mount is a staining technique used in the study of fungi to preserve and visualize fungal cultures. It is made by mixing a fungal culture with lactophenol and cotton blue, which penetrates the cell wall and binds to the cell contents, creating a permanent mount for observation and study.
What are the main applications of LPCB mounts?
LPCB mounts are used in a variety of applications in the study of fungal biology, including taxonomy and classification, the study of fungal diseases, and the study of fungal biochemistry and metabolism.
What are the benefits of using LPCB mounts?
LPCB mounts provide a permanent preservation of the fungal cells, which can be stored for an extended period of time for future reference and study. They also provide clear and easily visible staining of the fungal cells, making them easy to observe and study under a microscope.
What equipment is required to observe LPCB mounts?
Observing LPCB mounts requires a microscope, which can be expensive and may not be accessible to all researchers or institutions.
Can LPCB mounts be used for all types of fungi?
LPCB mounts can be used for a wide range of fungi, including yeasts, molds, and filamentous fungi.
What information can be obtained from LPCB mounts?
LPCB mounts provide information on the cellular structure and organization of fungal cells, which is important in the classification and identification of different types of fungi, as well as in the understanding of fungal biochemistry and metabolism.
Are there any limitations to using LPCB mounts?
LPCB mounts have some limitations and potential disadvantages, including the potential for staining artifacts and errors in interpretation, limited information on metabolic and biochemical processes, and the potential for sample degradation over time.
How is an LPCB mount made?
An LPCB mount is made by mixing a fungal culture with lactophenol and cotton blue, which penetrates the cell wall and binds to the cell contents. The resulting stained fungal cells are then placed on a microscope slide and covered with a coverslip.
Can LPCB mounts be stored for a long time?
Yes, LPCB mounts can be stored for an extended period of time, providing a permanent preservation of the fungal cells for future reference and study.
Are LPCB mounts a cost-effective method of preserving and visualizing fungal cultures?
Yes, LPCB mounts are a relatively cost-effective method of preserving and visualizing fungal cultures, making them accessible to a wide range of researchers and institutions.
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.