Lactic acid is manufactured commercially by both synthetic and fermentation processes. It is made synthetically in Japan and the United States. European manufacturers, on the other hand, make lactic acid by fermentation. European producers account for between 28,000 and 30,000 tonnes per year of the world’s estimated lactic acid production capacity. Pharmaceutical industries (e.g. iron lactate) and several plastics companies utilise lactic acid extensively.
What is Lactic Acid?
- Lactic Acid is a chemical compound having the formula C3H8O. It is also referred to as milk acid.
- When lactose (milk sugar) undergoes fermentation, lactic acid is produced.
- It is a component of cottage cheese, leban, sour milk, yoghurt, and Koumiss.
- Levo and Dextro are two kinds of lactic acid optical isomers.
- In 1780, Swedish chemist Carl Wilhelm Scheele extracted lactic acid from sour milk for the first time.
- The solid form of this chemical is water-soluble and white, but the liquid form is colourless.
- Calcium lactate, a soluble salt of lactic acid, can be utilised as a source of calcium.
- 1 mM of lactic acid has a pH of approximately 3.51.
Properties of Lactic Acid – C3H6O3
| C3H6O3 | Lactic Acid |
| Molecular Weight/ Molar Mass | 90.08 g/mol |
| pH | 3.51 |
| Boiling Point | 122 °C |
| Melting Point | 53 °C |
Chemical Structure of Lactic Acid
- It has an acid functional group (-COOH) and a hydroxyl functional group (-OH).
- Despite the presence of a hydroxyl functional group, it has higher acidic characteristics.
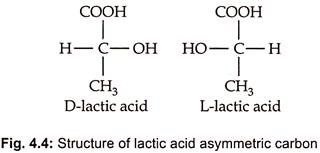
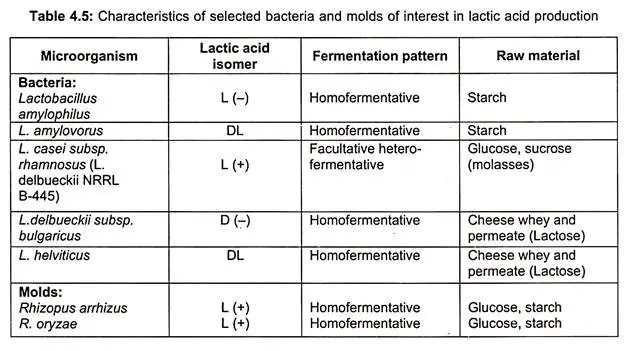
- Due to the presence of an asymmetrized carbon atom, lactic acid occurs in two forms: L (+) lactic acid and D-lactic acid.
- L-lactic acid is advantageous since it is digested by animal and human bodies, whereas the D- version is not metabolised and is primarily excreted.
- In commercial fermentations, however, a racemic mixture of both D and L is produced.
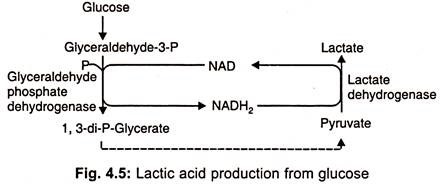
Biosynthesis of Lactic Acid
- Through glyceraldehyde-3-phosphate, 1, 3-diphosphoglycerate, and pyruvate, lactic acid is synthesised from glucose.
- During the oxidation of glyceraldehyde, reducing power is created.
- NAD-dependent lactate dehydrogenase transfers phosphate to pyruvate, which decreases stereospecifically to L (+) or D (-) lactic acid.
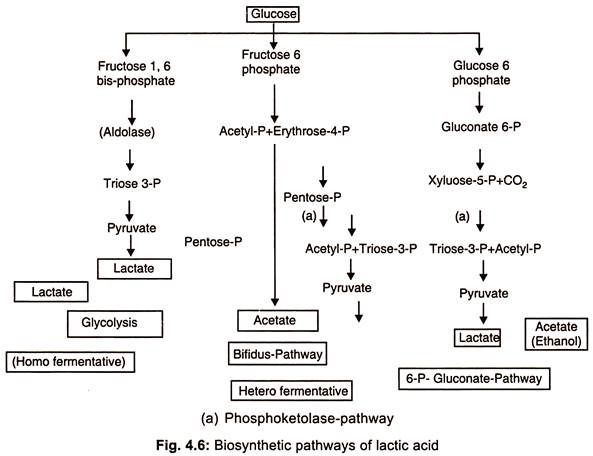
Fermentation Process of Lactic Acid
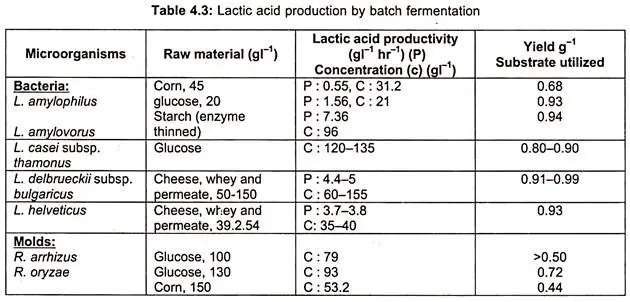
- Lactic acid is produced by both homofermenters such as Lactobacillus bulgaricus, L. plantarum, L. delbrueckii, Streptococcus lactis, and Pediococcus sp. and heterofermenters such as leuconostoc mesenteroides.
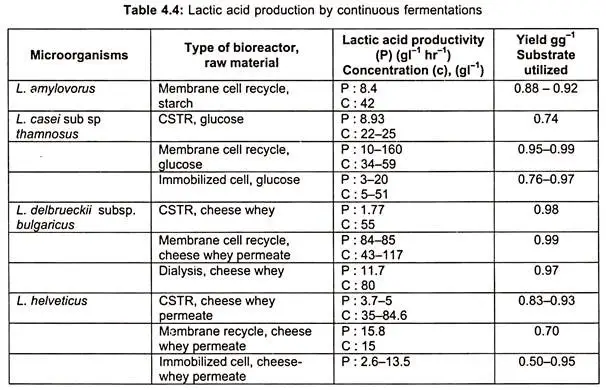
- In tables, the characteristics of bacteria and fungi that produce lactic acid via batch and continuous fermentation, as well as organisms used, bioreactor type, raw material employed, and lactic acid yield, are specified.
1. Inoculum Production
- Lactobacillus species are the microorganisms employed in the synthesis of lactic acid. There are two recognised forms of lactic acid bacteria: heterofermentative and homofermentative species.
- Therefore, heterofermentative organisms are not utilised in industrial processes.
- Therefore, homofermentative organisms are utilised in lactic acid fermentation because they generate fewer byproducts and more lactic acid.
- The selection of Lactobacillus species is heavily influenced by the carbon source employed during fermentation.
- When glucose is used as a substrate, Lactobacillus delbrueckii and Lactobacillus leichmannii are employed, while Lactobacillus bulgaricus and Lactobacillus pentosus are used when whey and sulfite waste liquor are used, respectively.
- Because these organisms are facultative anaerobes and not obligate anaerobes, bioreactors do not require complete oxygen exclusion.
- Suspension of pure bacterial culture is generated using a strain of high-yielding stock culture.
- The chosen Lactobacillus culture is moved to a big vessel, and the temperature is maintained between 45 and 50 degrees Celsius.
- Depending on the volume of fermentation broth, each culture-building stage requires 16 to 18 hours. Approximately 5% of the inoculum volume is typically consumed during fermentation.
2. Preparation of Medium
- To address the nutritional needs of the microorganisms, the medium must be formulated to include carbon source, nitrogen source, mineral source, and growth factor supply.
- Carbon sources include glucose, maltose, lactose, etc., or crude substrates such as corn starch, potato, rice, whey, molasses, sulfite waste liquor, etc.
- Pretreatment of starchy material with amylase or sulphuric acid is required for hydrolysis and the conversion of complicated starch into simple sugars such as glucose and maltose.
- If sulfite waste liquor is utilised in the medium, it is necessary to strip the sulfite using steam to convert it to sulphur dioxide and then treat it with alkali to eliminate the lysis during the pretreatment procedure.
- The sugar level of the medium is kept between 5 and 12 percent. Although several ammonium salts may be utilised, ammonium hydrogen phosphate is often used as the nitrogen source in lactic acid fermentation.
- Add approximately 0.25 percent ammonium salts to the medium. Minerals are given to the medium based on the selected microorganism’s nutritional needs. Calcium carbonate is also added as a pH-regulating buffering agent.
- For optimum growth, Lactobacillus requires vitamins, specifically vitamin B complexes. These are supplied to the medium as crude vegetable sources, such as the rootlets of growing barley seeds.
3. Fermentation Process
- In fermenters ranging from 25 to 125 klt, lactic acid is produced. Due to the corrosive nature of lactic acid, hardwood fermenters are utilised commercially rather than metal fermenters.
- Using calcium carbonate, the pH of the fermentation broth is kept between 5.5 and 6.5. Maintenance of pH is vital since it aids in both the acceleration of fermentation and the enhancement of yield.
- Low pH levels aid in both the removal of bacterial contamination and the sterilisation of substances at low temperatures.
- As lactic acid levels rise, it may become toxic to the organism. Therefore, accumulating acid is neutralised by the constant addition of calcium hydroxide. Typically, fermentation lasts between 5 and 10 days.
- It can also be completed in 72 hours if the medium contains 12 to 13% glucose and the pH is continuously maintained between 6.3 and 6.5 by neutralising the acid produced.
- However, total air deprivation is also not recommended because the bacteria used are facultative aerobes.
- To maintain the consistent distribution of nutrients and air, the fermenting broth is gently stirred.
- Despite the fact that the temperature is often maintained between 45 and 50 degrees Celsius, the precise temperature range varies to some extent on the organism used.
- For example Lactobacillus delbrueckii and L.bulgaricus demands 45-50°C, but L.casei and L.pentosus requires 30°C.
- Theoretically, one molecule of glucose produces two molecules of lactic acid. In actuality, this potential yield is exceeded by more than 90 percent. The lactic acid manufactured commercially is of the L (+) type.
4. Harvest and Recovery
- Technical or crude grade is coloured and available in 22, 44, 50, 60, and 80% concentrations, whereas edible grade is straw-colored and marketed at 50 to 80% concentrations of colourless high purity lactic acid, the plastic grade at 50 to 80% concentrations, and U.S.P. lactic acid at 85% concentrations.
- Other commercial lactic acid preparations include calcium lactate, sodium lactate, and copper lactate. The recovery procedure differs based on the needed grade of lactic acid.
Nonetheless, the steps of a broad strategy of recovery are detailed here:
- Using porcelain filters, the fermentation broth is filtered to remove the bacteria.
- The filtrate is acidified with sulfuric acid to regenerate lactic acid, rinsed, and calcium is precipitated as calcium sulphate.
- Activated carbon is added to the filtrate to eliminate organic contaminants.
- Steps of repeated refining and evaporation are required to obtain lactic acid concentrations of 50-60%.
- The material is subsequently treated with ferrocyanide to eliminate any heavy metals, such as copper.
- Finally, the water is cleaned by passing through ion exchange resins.
Other methods for extracting and purifying lactic acid include:
- preparing zinc salt of lactic acid, which has low solubility and can be recovered;
- directly extracting lactic acid with isopropyl ether using the counter-current method; and
- preparing methyl ester of lactic acid, which is easily hydrolyzed by boiling in water. High vacuum distillation of lactic acid is used to recover methyl alcohol.
Lactic Acid (C3H6O3) Uses
- It is utilised in food and medicine.
- It is used to manufacture polymers.
- It aids in the coagulation of milk proteins.
- It is utilised in the textile and leather tanning industries.
- Lactate is essential for the recovery of traumatic brain injuries.
- Lactate signals the release of norepinephrine in the brain.
- It is utilised in the production of surgical suture threads that dissolve on their own.
- As a blood coagulant and calcium source for the diet.
- In the production of cellophane resins, biodegradable polymers, as well as certain herbicides and pesticides.
- It is a mild acid with excellent solubility. It is a preservative used in the food business.
- Ethyl lactate is utilised in the production of anti-inflammatory medications.
Bioparameters Effects lactic acid manufacture
During the fermentation process of lactic acid production, the following bioparameters must be regulated:
1. The microorganism
- Numerous microbial species are capable of creating significant volumes of lactic acid. These include Lactobacillus bulgaricus, L. delbrueckii, L. leichmanni, L. casei, L. pentosus, Streptococcus lactis and Rhizopus oryzae.
- The selection of a microorganism for the fermentation process is heavily influenced by the carbon source employed. L. bulgaricus is favoured over other microbial species when whey is utilised as a carbohydrate source, for instance.
- Typically, the inoculum volume is around 5% of the volume of the fermentation broth.
2. Carbon sources
- As an acceptable supply of carbon, glucose, maltose, sucrose, or lactose may be utilised. As carbon sources in the production medium, crude substrates such as maize starch, potato starch, rice starch, whey, molasses, and sulfite lyes have been utilised.
- Before starch can be hydrolyzed into maltose and glucose, it must be pretreated with enzymes or an acid (such as sulfuric acid) beforehand.
- During the pre-treatment process, sulfite lyes must be stripped with steam to reduce sulphur dioxide and treated with alkali to remove lignin.
- Artichokes from Jerusalem are a possible raw material for lactic acid synthesis. Initially, the sugar concentration in the production medium is regulated to between 5 and 20 percent, but seldom exceeds 12 percent.
3. Nitrogen sources
- The usage of ammonium salts has shown satisfactory results. For instance, 0.25 percent ammonium hydrogen phosphate is used in the manufacturing of lactic acid in Hammond, Indiana, United States.
4. Growth factors and mineral sources
- Lactobacilli require complex nutrients, particularly vitamin B-complexes. These can be provided by supplementing the medium with crude vegetable sources (e.g. malt sprouts, i.e. the rootlets of germinating barley grains).
- This explains why care must be taken not to overheat the enrichment materials during the drying process in order to prevent any nutritional deficiencies.
- The production medium is supplemented with minerals, based on the needs of a specific microorganism.
- As a buffer, a high concentration (10 percent) of calcium carbonate is utilised.
5. pH
- A broth with a pH between 5.5 and 6.5 is deemed acceptable. It is crucial to regulate the pH since yields and rates can be boosted by doing so.
- The accumulation of acid is neutralised by the constant addition of a slurry of calcium hydroxide. If the acid is not neutralised, the consequence will be high acidity.
- Such high acid concentrations are intolerable to lactobacilli. Consequently, fermentation is incomplete.
- Such a fermentation medium with a low pH value is helpful since it eliminates the majority of contamination issues and permits sterilising at low temperatures.
6. Temperature
- The temperature maintained during fermentation is determined by the microorganisms involved. In fermentations including Lactobacillus bulgaricus or L. delbrueckii, for instance, a temperature range between 45 and 50°C has been determined to be optimal.
- Alternatively, employing L. casei, L. pentosus, or Streptococcus lactis, a temperature of approximately 30°C is deemed acceptable.
7. Aeration and agitation
- Since lactobacilli fermentation is anaerobic, the addition of sterile air is not required.
- In addition, total air exclusion is unnecessary because these bacteria are facultative aerobes. Typically, the broth is swirled gently to maintain the calcium carbonate suspended.
8. Time
- Typically, a plant fermentation lasts between 5 and 10 days. Using 12 to 13 percent glucose in the medium, fermentation can be completed in 72 hours if the pH is maintained between 6.3 and 6.5 through constant neutralisation of the acid generated.
9. Yield
- The yields of commercial fermentation range between 93 and 95% of the glucose given.
10. Recovery
- Product recovery, not fermentation, poses the greatest difficulty in lactic acid synthesis. The approach chosen for recovery is determined by the desired grade.
- Different types of grades are required for various objectives. These include food-grade, technical-grade, and plastic-grade materials.
- For food-grade lactic acid generation, a medium with a higher-quality sugar source and mineral protein content is required.
- Filtration using typical industrial precoat filters is the initial stage. For the regeneration of lactic acid, sulphuric acid is added to the filtrate.
- It is precipitated as calcium sulphate and cleaned. This wash is blended with the filtrate and then treated with activated vegetable carbon to eliminate organic contaminants.
- Following this, crude lactic acid is concentrated to 25 percent solids by evaporation. Repetition of the refining and evaporation processes yields 50 to 65 percent overall acidity.
- If the finished product is coloured, sodium ferrocyanide can be used to precipitate out heavy metals, such as copper. Using ion-exchange resins, the final remnants of pollution are eliminated.
- Precipitation removes calcium from technical-grade products as calcium sulphate dihydrate. The precipitate of calcium sulphate dihydrate is subsequently separated using filtration.
- Finally, the filtrate is evaporated to a concentration of 35 to 40 percent lactic acid. If additional calcium sulphate precipitates, it is also filtered out.
- Concentrating lactic acid and then esterifying it with methanol yields lactic acid suitable for plastics.