The Kinyoun-Cold Method is the cold acid-fast staining technique used for detecting acid-fast microorganisms, especially the species of Mycobacterium. It is a modified form of the Ziehl–Neelsen method, but in this method the application of heat is not required for staining. It is the reason why it is referred to as the cold method. It is the process in which the penetration of the primary stain occurs due to higher concentration of basic fuchsin and phenol, so the dye can enter through the waxy mycolic acid layer of the cell wall. The high phenol content increases the permeability of the cell wall and helps in retaining the stain during the cold incubation period.
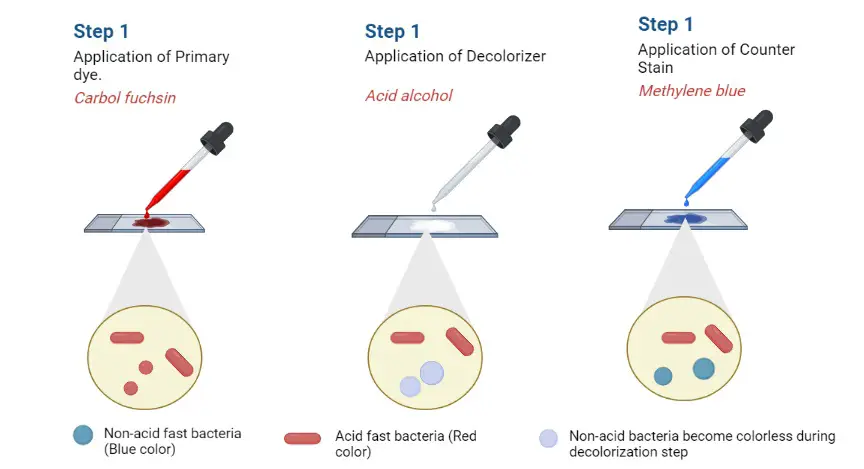
In this staining method, the smear is first treated with Kinyoun carbol fuchsin where the dye binds tightly with the lipid rich cell wall. After this step, strong acid-alcohol is used as decolorizer. Acid-fast bacteria resist this decolorization process because of the mycolic acid layer, so they appear bright red. Non-acid-fast cells lose the stain and these are counterstained with methylene blue or brilliant green, which gives a blue or green background.
It is used as an important method because it avoids the production of harmful phenolic fumes that occur when heating is used in Ziehl–Neelsen staining and it is more suitable in routine laboratory conditions. A modified Kinyoun method is also present where a weaker decolorizer like 1% sulfuric acid is used, and this method helps in staining weakly acid-fast organisms such as Nocardia spp. and some parasitic oocysts like Cryptosporidium, Isospora and Cyclospora.
Purpose of Kinyoun stain
The purpose of Kinyoun’s Acid Fast stain is to detection of acid-fast Mycobacterium spp. and other parasites i.e Cryptosporidium and Isopora spp.
Principle of Kinyoun-Cold Method
The principle of the Kinyoun-Cold Method is based on the ability of acid-fast bacteria to retain the primary stain even after strong decolorization. It is the process where the waxy cell wall of these bacteria, which contains a high amount of mycolic acid, becomes the major factor for differential staining. These organisms do not take up ordinary aqueous stains easily, so a concentrated carbol fuchsin solution is used. In this method, basic fuchsin and phenol are present in higher concentration, and the phenol acts as a chemical agent that increases permeability of the lipid-rich wall. It allows the dye to enter inside the cell without heating.
The carbol fuchsin forms a stable complex with the mycolic acid, and this complex is not removed even when treated with acid-alcohol. In this step the acid-fast cells remain red because the dye is tightly bound. Non-acid-fast organisms do not have this lipid layer, so the stain is removed from them during decolorization. After this treatment, a counterstain like methylene blue or brilliant green is applied, and these cells take up the secondary stain. This gives a clear contrast where acid-fast cells appear red and the background and non-acid-fast cells appear blue or green.
Requirements
- Kinyoun carbol fuchsin stain containing higher concentration of basic fuchsin and phenol.
- Acid–alcohol used as the standard decolorizer for removing primary stain from non-acid-fast cells.
- A mild decolorizer like 1% sulfuric acid can be required for weakly acid-fast organisms.
- Counterstains such as methylene blue or brilliant green used for giving contrast to non-acid-fast cells.
- Distilled water or deionized water used for proper rinsing of slides.
- Clean and grease-free glass slides used for preparing the smear.
- A permanent marker or pencil used for labeling the slides.
- Bright-field microscope with 100× oil immersion objective used for observation.
- Immersion oil used during microscopic examination.
- A staining rack or tray used for holding slides separately during staining.
- Slide warmer or air-drying used for drying the smear.
- Protective gloves and goggles used for safety during staining.
- Clinical specimens like sputum or stool sediment used for detecting acid-fast organisms.
- A thin and even smear prepared to ensure proper stain penetration.
- Smear is air-dried and then heat-fixed or chemically fixed before staining.
- A positive control smear containing known acid-fast organisms included in the staining set.
- A negative control smear containing non-acid-fast organisms used to check decolorization.
- Fresh and properly stored reagents used, and precipitated stains filtered before use.
- Examination of the smear performed by an experienced observer.
Procedure of Kinyoun staining
I. General Specimen Preparation and Fixation
Some of the main steps are–
- Label and Prepare Smear– It is done on a clean slide and a thin smear is prepared from sputum, culture or sediment. When solid culture is used then distilled water is taken to make the emulsion.
- Air Dry– The smear is allowed to air dry completely.
- Fix Smear– The smear is fixed either by passing the slide three times over flame or by using absolute methanol for about 30 seconds to 1 minute.
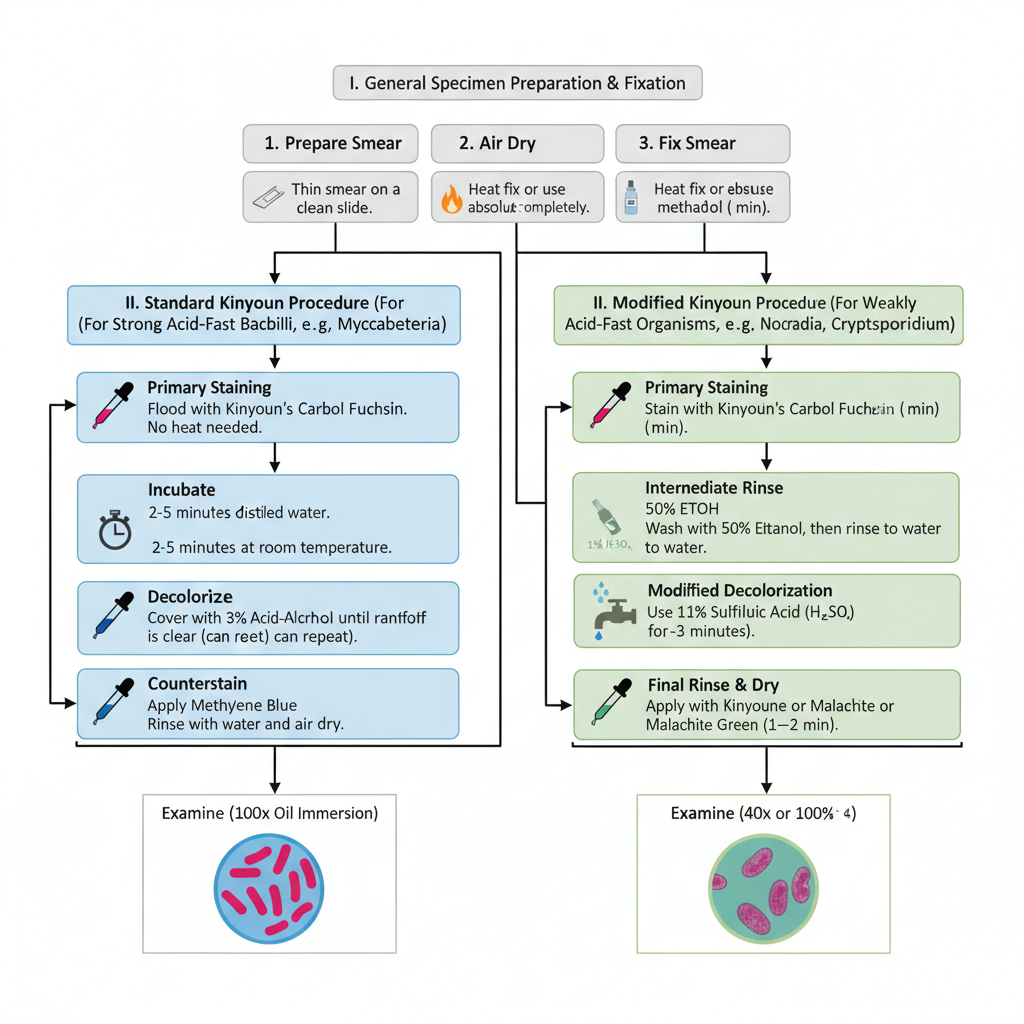
II. Standard Kinyoun Procedure (For Strong AFB/Mycobacteria)
In this step, a strong acid-alcohol is used as the decolorizer.
- Primary Staining– The smear is flooded with Kinyoun carbol fuchsin and no heat is required.
- Incubation– The stain is allowed to remain for about 2–5 minutes at room temperature.
- Rinse– It is rinsed gently with distilled water.
- Decolorization– The slide is covered with acid-alcohol solution (3% HCl in ethanol).
- Timing and Rinse– Decolorization is continued until the runoff becomes almost clear. This may take 3–5 seconds or sometimes up to 3 minutes. The smear is rinsed with water.
- Repeat Decolorization (If needed)– It is again decolorized for about 1–2 minutes until no more red stain appears, then rinsed properly.
- Counterstain– Methylene blue or Brilliant green is added for 30 seconds to 4 minutes.
- Final Rinse and Dry– The slide is rinsed with water and allowed to air dry. Blotting is avoided in some protocols.
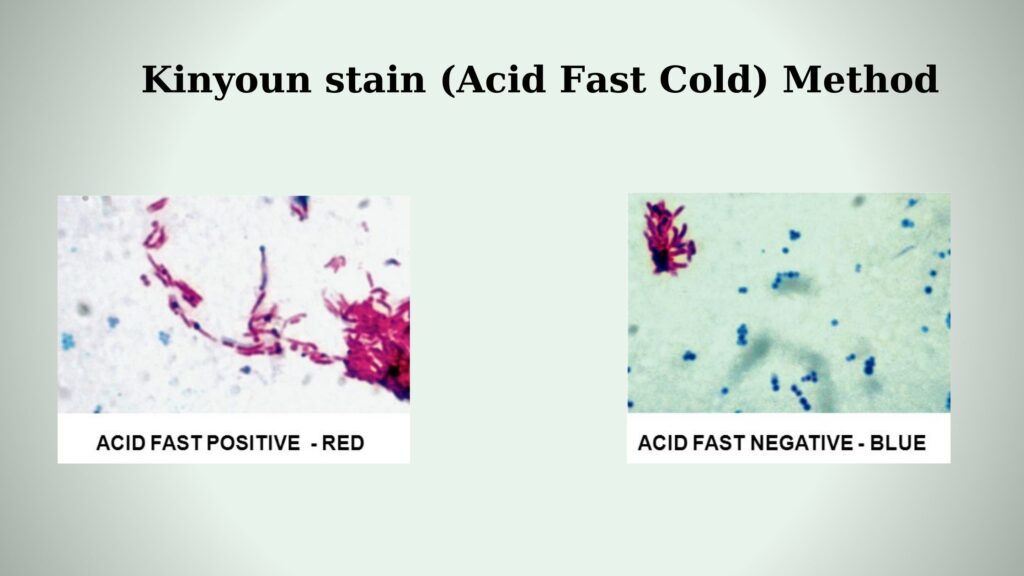
- Examine– It is examined under oil immersion (100×). Acid-fast organisms is seen as purple, red or pink.
III. Modified Kinyoun Procedure (For Weakly Acid-Fast Organisms/Oocysts)
This process occurs when organisms like Nocardia, Cryptosporidium or Isospora is to be detected using mild decolorizer.
- Fixation– The smear is fixed with absolute methanol for about 30 seconds to 1 minute.
- Primary Staining– The smear is stained with Kinyoun carbol fuchsin for 5 minutes at room temperature (sometimes 1 minute for coccidian oocysts).
- Intermediate Rinse– The slide is washed briefly with 50% ethanol for 3–5 seconds and then rinsed in water.
- Modified Decolorization– Decolorization is done using 1% sulfuric acid (H₂SO₄) for about 2–3 minutes until no more color comes off.
- Rinse– The slide is rinsed thoroughly with water.
- Counterstain– Methylene blue or Malachite green/Light green is applied for 1–2 minutes.
- Final Rinse and Dry– It is rinsed with water and dried either by air or blotting.
- Examine– The smear is examined under high power (40×) or oil immersion (100×). These organisms stain pink, red or purple.
Result of Kinyoun staining
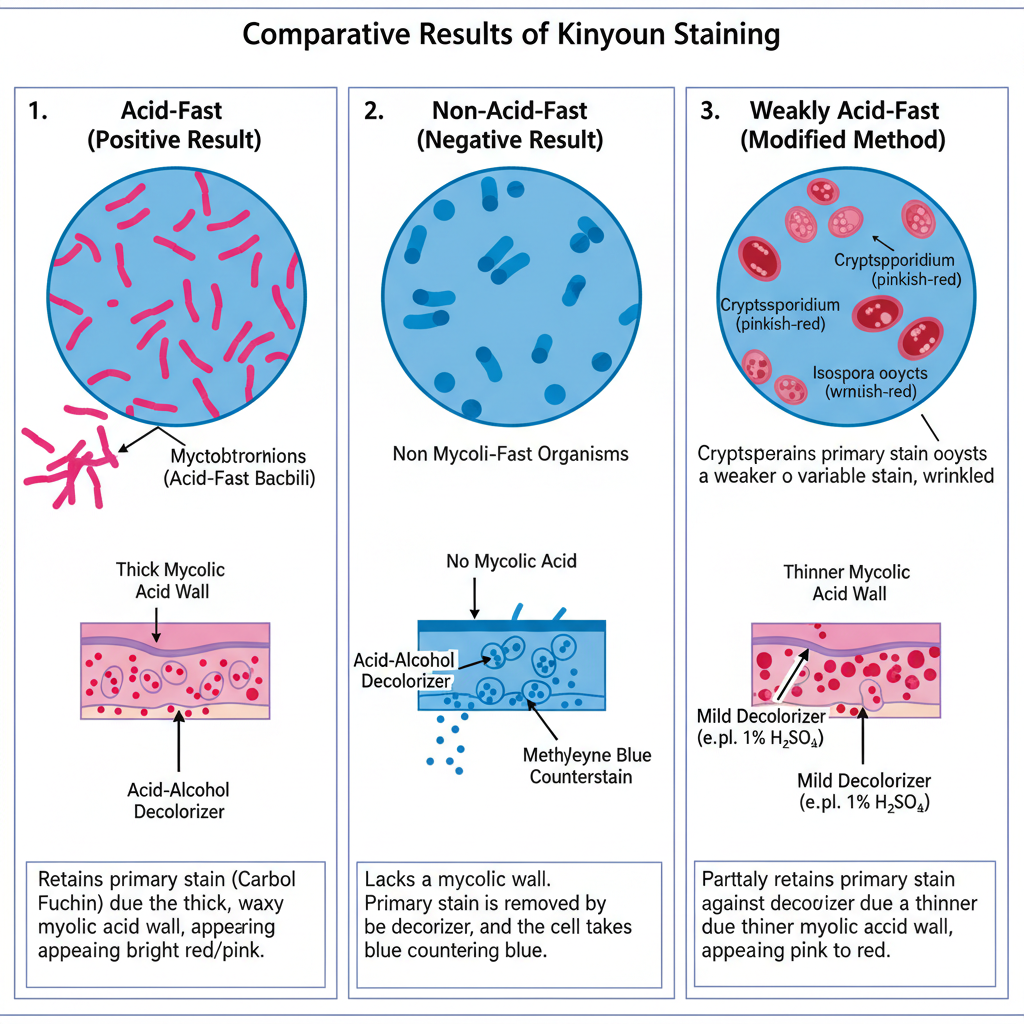
Acid-Fast Organisms (Positive Result)
It is observed that acid-fast organisms retain the primary stain and appear bright red, pink, or sometimes purple. The color is held strongly because the mycolic acid of the cell wall does not allow the acid-alcohol to remove the carbol fuchsin. This property is referred to as acid-fastness.
These organisms is generally slender bacilli measuring about 1–10 μm, and they may be slightly curved in some fields. In many smears, the bacilli show beaded appearance where some parts stain more intensely. Some species may also show pleomorphic long filaments or coccoid forms with uniform staining.
Among the important organisms detected are Mycobacterium species like M. tuberculosis and M. leprae. The presence of even a single acid-fast bacillus in a clinical smear is considered significant.
Non-Acid-Fast Organisms (Negative Result)
Non-acid-fast organisms appear blue or green according to the counterstain used. It is because these organisms do not have the lipid-rich wall, and the acid-alcohol removes the primary stain completely. After decolorization, the cells take up methylene blue or brilliant green and appear in the background.
The background debris and other normal flora also appear in the color of the counterstain.
Modified Kinyoun Result (Weakly Acid-Fast Organisms)
This process occurs when a mild decolorizer like 1% sulfuric acid is used. Weakly acid-fast organisms stain pink, red or deep purple.
Cryptosporidium oocysts are seen as round bodies measuring about 4–6 μm and stain pinkish red. Isospora oocysts appear red. Cyclospora oocysts show variable staining from light pink to deep red and sometimes show granular or bubbly content which is described as wrinkled cellophane appearance.
The background and non-acid-fast structures stain blue or green depending on the counterstain used.
Uses of Kinyoun staining Method
Some of the important uses are–
- It is used for identification of acid-fast organisms having waxy cell walls.
- It helps in rapid screening and presumptive diagnosis of pulmonary tuberculosis.
- It is suitable for demonstration of Mycobacterium tuberculosis in sputum, tissues, and FNAC samples.
- It is used to detect other mycobacterial diseases including M. leprae and non-tuberculous mycobacteria.
- It is applied for checking the presence of acid-fast bacilli in patients receiving treatment and helps in monitoring therapy response.
- The modified method is used for weakly acid-fast bacteria such as Nocardia species.
- It is helpful in detecting coccidian oocysts like Cryptosporidium, Isospora, and Cyclospora.
- Some organisms like Rhodococcus, Gordonia, Actinomadura and Tsukamurella also retain the dye and can be identified.
- It is used as an alternative to the Ziehl–Neelsen method for primary diagnosis of tuberculosis.
Limitations of Kinyoun staining Method
Some of the main limitations are–
- It is less sensitive as compared to the Ziehl–Neelsen method.
- A high number of organisms is required because smear detection needs about 5,000–10,000 AFB per mL.
- The method has limited specificity since all mycobacteria are acid fast and species identification is not possible.
- It is the process which does not differentiate viable and dead bacilli, so treated patients may show smear positive but culture negative.
- Smear examination is lower in sensitivity than culture techniques for diagnosing mycobacterial infection.
- Light infections with very few oocysts can be missed.
- Thick smears do not stain or destain properly and they may flake during staining.
- Very thin smears may not contain enough sample which can result in false negative reports.
- A minimum of 300 oil immersion fields is needed to be examined before reporting a smear as negative.
- Carbol fuchsin precipitates on standing and the deposits can be mistaken as organisms causing false positive result.
- The quality of reagents must be maintained because methylene blue and decolorizer degrade easily if not stored properly.
- AAT Bioquest. (2023, March 22). What are the fundamental differences between Ziehl-Neelsen technique and the Kinyoun technique?
- AAT Bioquest. (2023, March 22). What are the limitations of the Kinyoun-cold method?
- Association of Public Health Laboratories. (n.d.). AFB smear microscopy (Essentials of the Mycobacteriology Laboratory: Promoting Quality Practices).
- Aryal, S. (2021, July 27). Acid Fast Stain (Kinyoun-Cold Method)- Principle, Procedure and Result Interpretation. Microbe Notes.
- Bayot, M. L., Mirza, T. M., & Sharma, S. (2023). Acid Fast Bacteria. In StatPearls [Internet]. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK537121/
- Centers for Disease Control and Prevention. (2016). Stool specimens – Staining procedures. DPDx – Laboratory Identification of Parasites of Public Health Concern.
- Chopra, S., Mahajan, S., Singh, Y., & Chopra, M. (2022). Comparative evaluation of Ziehl- Neelsen staining and kinyoun’s staining in the diagnosis of clinically suspected cases of tuberculosis. IP International Journal of Medical Microbiology and Tropical Diseases, 8(2), 149–153. https://doi.org/10.18231/j.ijmmtd.2022.031
- Dalynn Biologicals. (2014). KINYOUN CARBOL FUCHSIN STAIN (Catalogue No. SK50).
- Ethos Biosciences. (2025). Fix an acid-fast organism that’s not retaining dye.
- Ethos Biosciences. (2025). How to set-up and conduct an acid fast stain.
- Hartline, R. (2023). 1.14: Acid-Fast Stain. In Microbiology Laboratory Manual. Biology LibreTexts.
- Kaiser, G. (2023). 2.3C: The Acid-Fast Cell Wall. In Microbiology. Biology LibreTexts.
- Kurup, R., & Chester, K. (2014). Comparative evaluation of Ziehl Neelsen staining and knowledge, attitudes and practices of laboratory personnel in relation to Ziehl Nielsen. West Indian Medical Journal, 63(1), 34–39. https://doi.org/10.7727/wimj.2012.247
- Leica Biosystems. (n.d.). Acid Fast Bacteria and Acid Fast Staining.
- MacKenzie, E. (2025). 23: ACID FAST STAIN. In IVC Microbiology Lab Manual. Biology LibreTexts.
- Medical Chemical Corporation. (n.d.). MODIFIED KINYOUN’S ACID-FAST STAIN (COLD).
- MI – Microbiology. (n.d.). Acid-Fast.
- Parker, N., Schneegurt, M., Tu, A. T., Lister, P., & Forster, B. M. (2016). 2.4 Staining Microscopic Specimens. In Microbiology. OpenStax. Retrieved from https://openstax.org/books/microbiology/pages/2-4-staining-microscopic-specimens
- Remel Inc. (2010). TB Kinyoun carbolfuchsin (IFU 40104). Thermo Fisher Scientific.
- Sigma-Aldrich. (n.d.). Carbol-Fuchsin solution according to Kinyoun.
- UK Health Security Agency. (2025). Staining procedures (UK Standards for Microbiology Investigations, Test Procedures TP 39, Issue 3.1). Royal College of Pathologists.
- Watson, R. (n.d.). Ziehl Neelsen Acid-fast stain. The Virtual Edge.