What is Karyotyping?
Karyotyping: What’s the Deal?
- Karyotyping checks out your chromosomes to find any genetic hiccups.
- Scientists have been doing this for a long time.
- It’s not as high-tech as PCR or DNA sequencing, but it’s still useful.
- To do karyotyping, you need to culture cells, stain them, and look at the bands on the chromosomes.
- It’s a bit old-school but still handy for spotting genetic problems.
- You can catch changes in chromosome number or structure, like deletions or duplications.
- Doctors use it to diagnose conditions like Down syndrome, Patau syndrome, and Edward’s syndrome.
- Originally, karyotyping was for plants, not humans, but now it’s used to check for human genetic issues.
- The term “karyotyping” comes from the Greek word “Karyon,” meaning nucleus.
- Back in 1842, Carl Wilhelm von Nageli spotted thread-like structures in plant cells and called them transitory cytoplasts—these were chromosomes.
- In 1888, Waldeyer named these structures chromosomes.
- By 1956, scientists Tjio and Levan found humans have 46 chromosomes, arranged in 23 pairs.
- Most of our DNA is in these chromosomes, and any changes can lead to genetic disorders.
- The main goal of karyotyping is to spot any chromosomal variations.
- Despite being less advanced than newer techniques, karyotyping is still a key tool in genetic diagnostics.
- It’s used to find genetic disorders, determine sex, and study evolutionary relationships.
- While not as common now, it’s crucial for deepening our understanding of genetics.
- Karyotyping’s been around for over half a century and still plays a vital role in medicine and research.
Definition of Karyotyping
Karyotyping is a diagnostic technique that examines an individual’s chromosomes to identify genetic abnormalities, structural changes, and chromosome number variations.
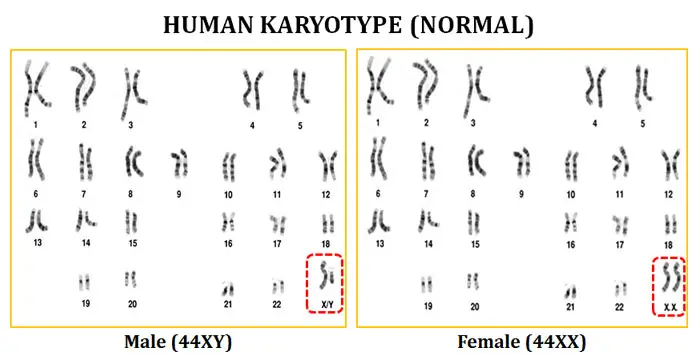
What is a karyotype?
- Definition: A karyotype refers to the organized arrangement of chromosomes in pairs to detect abnormalities.
- Preparation: After obtaining GTG banding results, a photograph is taken using a high-quality camera.
- Arrangement: The printed chromosomes are cut out individually and arranged sequentially on paper. Chromosomes 1 to 22 are sorted first, followed by the X and Y chromosomes.
- Visualization: To aid visualization, the arranged chromosomes are typically mounted on colored paper.
- Analysis: Interpreting karyotype results requires expertise and time compared to simpler techniques like agarose gel electrophoresis.
- Characteristics: Each chromosome pair exhibits unique characteristics such as size, centromere position, and banding patterns.
- Automated vs. Manual: Modern karyotyping software can rapidly arrange chromosomes computationally, selecting optimal metaphase fields. However, manual validation remains crucial for accuracy.
- Expertise: Significant experience in cytogenetics and karyotyping is essential for interpreting karyotype results effectively.
Karyotyping Procedure/Steps of Karyotyping
- Sample Collection: A sample from tissues, amniotic fluid, or blood is needed for karyotyping.
- Cell Culturing: The collected sample undergoes cell culturing in a nutrient-rich medium like RPMI-1640 under strict sterile conditions to prevent contamination.
- Metaphase Arrest: Colchicine is added to arrest cells during metaphase, a stage of cell division where chromosomes are highly condensed and visible.
- Chromosome Preparation: A hypotonic solution is used to swell and spread chromosomes on a slide for better visibility.
- Staining: Giemsa staining followed by GTG (Giemsa-trypsin-Giemsa) banding is applied to the slide to create distinctive dark and light bands on the chromosomes.
- Karyogram Creation: The stained chromosomes are arranged and photographed to create a karyogram, a visual representation of the individual’s chromosomal composition.
Staining and banding
Staining and Banding techniques used in karyotyping:
- Giemsa Staining:
- The prepared slide is immersed in a Giemsa solution for 10 to 15 minutes.
- This staining method helps in visualizing chromosomes under a 45X microscope.
- It provides a general view to ensure chromosomes are present and to check for any obvious abnormalities.
- GTG Banding:
- GTG (Giemsa-Trypsin-Giemsa) banding is widely used for identifying numerical and structural chromosome variations.
- Trypsin digests chromosome proteins, exposing loosely packed regions.
- Giemsa stains these regions, creating distinct light and dark patterns on chromosomes.
- Dark bands represent heterochromatin (densely packed DNA), while light bands indicate euchromatin (less densely packed DNA).
- Q-Banding:
- Q-Banding utilizes quinacrine fluorescent dye to highlight AT-rich heterochromatic regions.
- The dye emits yellow fluorescence under a fluorescent microscope, revealing distinct banding patterns on chromosomes.
- It is similar to GTG banding but uses fluorescence for visualization.
- C-Banding:
- C-Banding selectively stains centromeric regions of chromosomes.
- This technique is different from GTG banding and is used specifically to study centromeric structures.
- It is named for its focus on the centromere (“C” stands for centromere).
- R-Banding:
- R-Banding, or reverse banding, is the opposite of G-Banding.
- Here, euchromatin regions appear darker and heterochromatin regions appear lighter compared to G-Banding.
- It provides an alternative perspective on chromosome structure and organization.
- NOR-Banding:
- NOR-Banding uses silver staining to detect nucleolar organizing regions on chromosomes.
- It is particularly useful for identifying acrocentric chromosomes that have specific genetic sequences related to ribosomal RNA production.
- T-Banding:
- T-Banding focuses on studying telomeric regions of chromosomes.
- It is a less commonly used technique compared to others but provides insights into chromosome end structures.
Applications of Karyotyping
Medical Genetics:
- Karyotyping is crucial in identifying chromosomal abnormalities linked to genetic disorders.
- It detects numerical anomalies such as trisomy 21 (Down syndrome) or monosomy X (Turner syndrome).
- Structural abnormalities like translocations or deletions, associated with diseases like leukemia or muscular dystrophy, can also be identified.
Prenatal Diagnostics:
- Karyotyping is used during prenatal diagnostics to detect chromosomal abnormalities in fetuses.
- Samples of amniotic fluid or chorionic villi are examined to identify potential genetic issues early in pregnancy.
Gender Determination:
- Karyotyping determines a person’s sex by analyzing the presence or absence of specific chromosomes.
- Males typically have one X and one Y chromosome, while females have two X chromosomes.
Evolutionary Biology:
- In evolutionary biology, karyotyping helps study species’ evolutionary relationships.
- By comparing karyotypes of different species, scientists trace evolutionary histories and divergence points.
- For example, humans have 23 pairs of chromosomes while chimpanzees have 24, reflecting evolutionary changes.
Cytogenetic Information:
- A karyotype provides cytogenetic information about chromosome number, shape, size, and any alterations.
- It is essential for diagnosing conditions caused by chromosomal abnormalities.
Observations and Techniques:
- While traditional karyotyping methods are time-consuming and prone to contamination, they remain essential for certain genetic analyses.
- Observations include determining chromosome numbers and identifying major deletions or insertions.
- Techniques like G-staining or GTG-banding are used to examine chromosome structure and identify abnormalities.
Chromosome Characteristics:
- Karyotyping reveals absolute and relative size variations among chromosomes.
- It identifies variations in centromere location, crucial for distinguishing between chromosome pairs.
- Banding techniques like NOR-banding or C-banding are used to study specific chromosome regions such as telomeres and satellite regions.
Botanical Applications:
- In plants, karyotyping detects aneuploidies and polyploidies, providing insights into genetic variations and evolution within plant species.
Limitations of karyotyping
Detection Scope:
- Karyotyping can only detect abnormalities related to the number or structure of chromosomes visible during metaphase.
- It does not identify epigenetic changes or mutations affecting gene expression.
Cell Availability:
- Sufficient cells are required for karyotyping, which may be limited in scenarios involving early embryos or fetal tissues.
Sex Determination:
- Karyotyping can determine sex based on the presence or absence of a Y chromosome.
- It may not provide definitive results for individuals with ambiguous genitalia or complex sex chromosome variations.
Evolutionary Studies:
- Karyotyping’s resolution with chromosomal bands may not distinguish subtle variations in chromosome structure or gene content.
- It does not explain convergent or parallel evolution, where similar traits evolve independently in different species.
Recent advances in karyotyping
- Fluorescence In Situ Hybridization (FISH):
- FISH uses fluorescent probes to target specific DNA sequences on chromosomes.
- It detects abnormalities with higher sensitivity compared to traditional karyotyping.
- FISH can identify minor deletions or duplications that may be missed by standard banding techniques.
- Comparative Genomic Hybridization (CGH):
- CGH employs microarrays to compare DNA content between samples, like healthy and cancerous cells.
- It detects copy number variations (CNVs) across the entire genome, providing a comprehensive view of genetic alterations.
- CGH is more sensitive than karyotyping in detecting genetic changes that affect large segments of DNA.
- Whole Genome Sequencing (WGS):
- WGS offers a detailed view of an individual’s entire genome, including chromosomes and genes.
- It identifies point mutations, structural alterations, and other genetic changes with high resolution.
- Despite its capabilities, WGS is currently more costly and time-consuming than traditional karyotyping.
- Spectral Karyotyping (SKY):
- SKY enhances traditional karyotyping by using multiple fluorescent dyes to color each chromosome pair uniquely.
- This method simplifies the observation and identification of chromosomal abnormalities.
- SKY is effective in detecting balanced and unbalanced translocations, deletions, and other complex genetic variations.
- Applications:
- Karyotype tests are crucial in prenatal screening for conditions like Down syndrome, Patau syndrome, and Edwards syndrome.
- Samples such as amniotic fluid or chorionic villi are cultured for karyotyping to detect chromosomal abnormalities.
- SKY and other advanced techniques complement traditional karyotyping, offering improved accuracy in genetic analysis.
Using Karyograms to Detect Chromosomal Abnormalities
Diagnostic Tool:
- G-banded karyograms are commonly used to diagnose various chromosomal abnormalities in individuals.
- They provide a visual map of an individual’s chromosomes, highlighting structural changes that may indicate genetic disorders.
Detecting Aneuploidy:
- Aneuploidy, characterized by the presence of an extra or missing chromosome, is easily identifiable through karyotyping.
- This includes conditions like Down syndrome, where an extra copy of chromosome 21 is present.
Identifying Deletions and Insertions:
- Karyograms can detect subtle deletions or insertions in chromosomes.
- Changes in banding patterns indicate deviations from the normal genetic structure.
Detecting Translocations:
- Translocations, where parts of chromosomes break and rearrange with other chromosomes, are visible on karyotypes.
- These structural changes are crucial in diagnosing disorders like chronic myelogenous leukemia.
Genetic Research and Critical Intervals:
- Researchers use karyograms to identify critical intervals on chromosomes where genetic changes may cause diseases.
- Advances from the Human Genome Project have linked cytogenetic bands with DNA sequences, aiding in pinpointing candidate genes.
Molecular Cytogenetic Techniques:
- Molecular cytogenetics, such as Fluorescence In Situ Hybridization (FISH) and Comparative Genomic Hybridization (CGH), offer higher resolution in detecting abnormalities.
- These techniques can pinpoint genetic changes at the level of individual genes, complementing traditional karyotyping.
Future Directions:
- As diagnostic technologies evolve, cytogeneticists are integrating new methods to enhance the precision and efficiency of genetic diagnoses.
- The transition from karyotyping to molecular techniques promises continued advancements in understanding and treating genetic disorders.
Preparing Karyotypes from Mitotic Cells
Karyotyping entails examining the quantity, make-up, and arrangement of chromosomes within a cell. It is a crucial tool in genetics and medical diagnostics, helping to detect chromosomal abnormalities. Mitotic cells, which are actively dividing cells, are essential for creating karyotypes due to their condensed chromosome structures during metaphase.
- Cell Selection and Culture:
- Mitotic cells are selected from various tissue types such as tumor biopsies, bone marrow, peripheral blood, skin biopsies, amniotic fluid, or chorionic villus samples for prenatal diagnosis.
- Cells are cultured briefly to allow them to proliferate and prepare for karyotyping.
- Mitotic Arrest:
- Colchicine, a chemical agent, is introduced to arrest cells in metaphase of the cell cycle.
- This phase ensures chromosomes are tightly condensed and visible for accurate analysis.
- Chromosome Preparation:
- After metaphase arrest, cells undergo treatment with a hypotonic solution to swell nuclei and facilitate chromosome release.
- Chemical fixatives are applied to preserve the chromosome structure when cells are spread on glass slides.
- Staining and Analysis:
- Chromosomes on the slides are stained using dyes like Giemsa, which highlight distinct banding patterns on the chromosomes.
- The banding patterns reveal structural details such as size, shape, and arrangement of chromosomes.
Banding Patterns Reveal the Structural Details of Chromosomes
Banding patterns are essential in cytogenetics for unveiling the intricate structure of chromosomes. These patterns arise from specialized staining techniques that highlight different regions of chromosomes, aiding in their identification and detailed analysis.
- G-banding Technique:
- G-banding involves staining chromosomes with Giemsa dye after brief treatment with trypsin.
- It highlights heterochromatic regions (AT-rich and gene-poor) as dark bands and euchromatic regions (GC-rich and transcriptionally active) as light bands.
- This technique provides reproducible patterns for each chromosome, crucial for diagnostic purposes.
- R-banding Technique:
- R-banding reverses the pattern of G-banding by heating chromosomes before staining.
- Heat treatment preferentially affects AT-rich regions, leaving GC-rich regions to take up Giemsa stain.
- It is valuable for studying gene-rich regions near telomeres.
- C-banding Technique:
- C-banding specifically stains constitutive heterochromatin at centromeres.
- It uses a specialized Giemsa technique to highlight AT-rich satellite DNA.
- Though less commonly used diagnostically now, it provides insights into chromosome structure.
- Q-banding Technique:
- Q-banding utilizes quinacrine staining under UV light to identify chromosomal translocations.
- Particularly useful for detecting abnormalities involving the Y chromosome.
- It was the first method to identify all 46 human chromosomes, pioneering modern cytogenetic analysis.
Conclusion
Karyotyping serves as a fundamental tool in evolutionary biology and medical genetics, providing a visual and analytical framework for studying chromosomes in individuals and species alike. Over the past five decades, it has been instrumental in identifying chromosomal abnormalities, determining sex chromosome composition, and exploring evolutionary relationships. Despite its advancements, karyotyping has limitations in cases of ambiguous genitalia or subtle genetic alterations beyond visible chromosomes during metaphase.
Advancements in molecular genetics and genomics have enhanced the precision and scope of karyotyping, yet they also introduce challenges in interpretation and application. Nonetheless, karyotyping remains indispensable in diagnosing genetic conditions and birth defects, enabling early intervention and personalized medical care. Today, automated systems equipped with powerful microscopes and sophisticated software ensure rapid, robust, and highly accurate results. However, the expertise of trained professionals remains essential for the accurate interpretation and reporting of karyotype results.
References
- Caspersson, T., Zech, L., & Johansson, J. Differential banding of alkylating fluorochromes in human chromosomes. Experimental Cell Research 60, 315–319 (1970) doi:10.1016/0014-4827(70)90523-9
- Gartler, S. M. The chromosome number in humans: A brief history. Nature Reviews Genetics 7, 655–660 (2006) doi:10.1038/nrg1917
- Speicher, M. R., Ballard, S. G., & Ward, D. C. Karyotyping human chromosomes by combinatorial multi-fluor FISH. Nature Genetics 12, 368–375 (1996)
- Bates SE. Classical cytogenetics: karyotyping techniques. Methods Mol Biol. 2011;767:177‐190.
- Schreck RR, Distèche C. Karyotyping. Curr Protoc Hum Genet. 2001; Appendix 4.
- https://www.nature.com/scitable/topicpage/karyotyping-for-chromosomal-abnormalities-298/