What is Isomerism?
- Isomerism is a fundamental concept in chemistry that refers to the existence or possibility of isomers. Isomers are molecules or polyatomic ions that have the same molecular formula, meaning they have the same number of atoms of each element, but they have distinct arrangements of atoms in space.
- The term “isomer” comes from the word “isomeric” and was borrowed from German “isomerisch,” which itself originated from the Swedish word “isomerisk.” The Swedish term was coined from the Greek word “ἰσόμερoς” (isómeros), which combines “isos” meaning “equal” and “meros” meaning “part.” This linguistic evolution reflects the underlying principle of isomerism, where molecules have the same parts (atoms) but are arranged differently.
- Isomers can be categorized into two main forms: structural or constitutional isomerism and stereoisomerism or spatial isomerism. Structural isomers have different arrangements of bonds between the atoms, while stereoisomers have the same bonds but differ in the relative positions of the atoms.
- It’s important to note that isomers do not necessarily exhibit similar chemical or physical properties. Even though they have the same molecular formula, their distinct arrangements in space can lead to different behaviors and characteristics. This diversity in properties is what makes isomerism a fascinating and important aspect of chemistry.
- Isomeric relationships can form a hierarchy, where different levels of analysis are required to identify and differentiate between various types of isomers. For example, two chemicals may initially appear to be the same constitutional isomer but could be found to be stereoisomers upon closer examination. Similarly, two molecules that are stereoisomers of each other may exhibit different conformational forms or be classified as different isotopologues, depending on the specific field of study or the chemical and physical properties of interest.
- In conclusion, isomerism is a concept in chemistry that encompasses molecules or polyatomic ions with the same molecular formula but distinct arrangements of atoms in space. It encompasses both structural and stereoisomerism, which involve differences in bond arrangements and relative positions of atoms, respectively. Isomerism highlights the versatility and complexity of chemical compounds and plays a crucial role in understanding their diverse properties and behaviors.
Definition of Isomerism
Isomerism is the phenomenon in chemistry where molecules or ions have the same molecular formula but differ in their spatial arrangement or bond connectivity.
Different Types of Isomerism
Isomerism, a fundamental concept in chemistry, can be classified into two primary types: structural isomerism and stereoisomerism. Let’s delve into these types and explore their various subtypes.
- Structural Isomerism: Structural isomerism, also known as constitutional isomerism, refers to isomers that have different arrangements of atoms and bonds. This type of isomerism can be further classified into the following subtypes:
- a) Chain Isomerism: In chain isomerism, the carbon skeleton of the molecule differs between isomers. This can involve variations in the length or branching pattern of the carbon chain.
- b) Position Isomerism: Position isomerism occurs when functional groups or substituents are attached to different positions on the carbon skeleton. The connectivity of atoms remains the same, but their placement within the molecule changes.
- c) Functional Group Isomerism: Functional group isomerism involves isomers with different functional groups. The molecular formula remains the same, but the type of functional group present varies between isomers.
- d) Tautomeric Isomerism: Tautomeric isomerism occurs when isomers interconvert rapidly due to the migration of a hydrogen atom and a double bond within the molecule. This results in different resonance structures and the ability to exist in different forms.
- Stereoisomerism: Stereoisomerism refers to isomers that have the same connectivity of atoms but differ in their spatial arrangement. This type of isomerism can be further categorized into the following subtypes:
- a) Geometric Isomerism: Geometric isomerism arises when molecules have restricted rotation around a bond and exist in different geometric configurations. The most common form of geometric isomerism is cis-trans isomerism, where substituents are positioned differently around a double bond or a ring.
- b) Optical Isomerism: Optical isomerism, also known as enantiomerism, occurs when isomers are non-superimposable mirror images of each other. These isomers are referred to as enantiomers and exhibit different optical activities.
- c) Conformational Isomerism: Conformational isomerism arises due to the rotation of single bonds, resulting in different spatial arrangements of the molecule. The different conformations are often interconvertible without breaking any bonds.
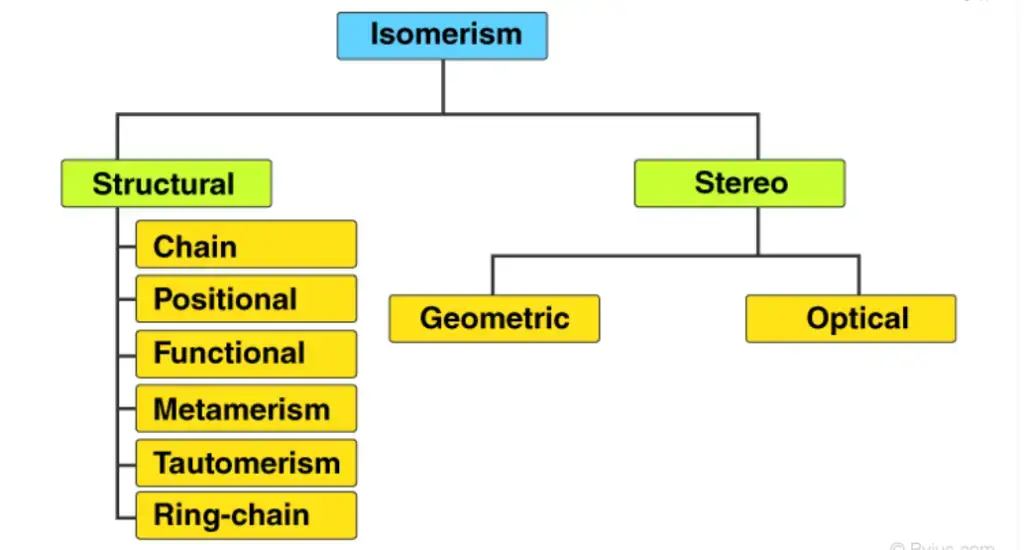
These are the primary types and subtypes of isomerism in chemistry. Each type of isomerism showcases unique variations in the arrangement of atoms and bonds or in the spatial orientation of molecules, leading to diverse properties and behaviors. Understanding these different types of isomerism is crucial for comprehending the intricate nature of chemical compounds and their interactions.
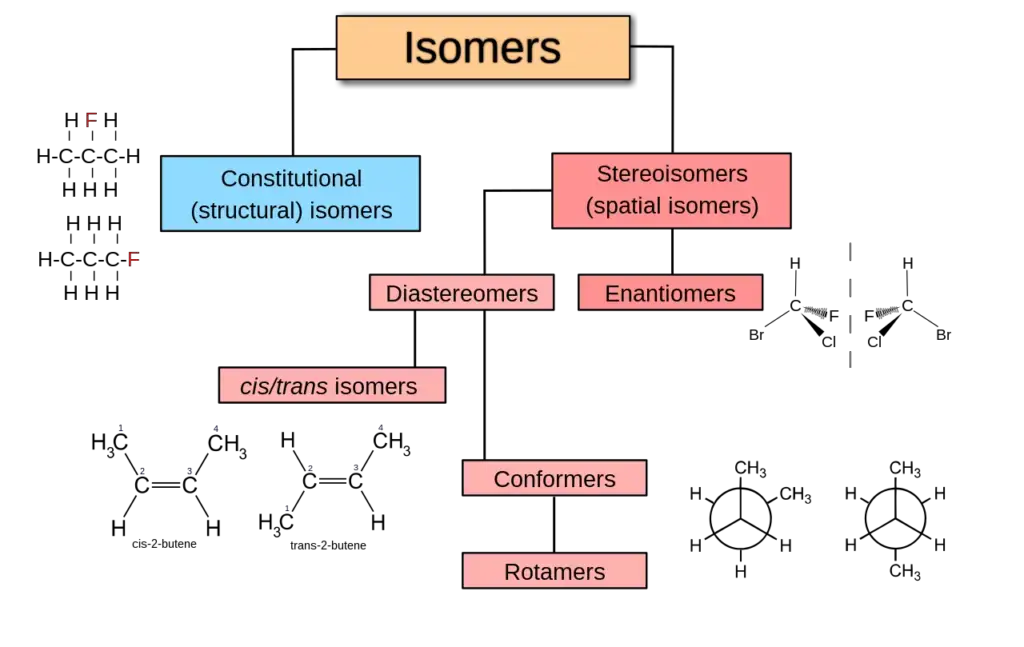
A. Structural Isomerism
Structural isomerism, also known as constitutional isomerism, is a type of isomerism where molecules have the same molecular formula but differ in the connectivity of atoms and bonds. This leads to distinct arrangements of atoms within the molecule. Here are a few examples to illustrate the concept of structural isomerism:
Example: C3H8O For the molecular formula C3H8O, there are three structural isomers:

- Propan-1-ol (1-propanol, n-propyl alcohol, n-propanol): In this isomer, the hydroxyl group (-OH) is attached to the first carbon atom of the three-carbon chain. The condensed structural formula is H3C-CH2-CH2OH.
- Propan-2-ol (2-propanol, isopropyl alcohol, isopropanol): In this isomer, the hydroxyl group (-OH) is attached to the second carbon atom of the three-carbon chain. The condensed structural formula is H3C-CH(OH)-CH3.
- Methoxyethane (ethyl-methyl-ether): This isomer has the oxygen atom connected to two carbon atoms, with all eight hydrogens bonded directly to carbons. The condensed formula is H3C-O-CH2-CH3.
Example: C3H4 For the hydrocarbon C3H4, there are three structural isomers:

- Propadiene (allene): In this isomer, the three carbon atoms are connected by two double bonds. The remaining valences of the carbon atoms are satisfied by four hydrogens. The structure can be represented as H2C=C=CH2.
- Propyne (methylacetylene): In this isomer, the three carbon atoms are connected by a single bond and a triple bond. The remaining valences of the carbon atoms are satisfied by four hydrogens. The structure can be represented as HC≡CCH3.
- Cyclopropene: This isomer forms a cyclic structure where the three carbons are connected in a ring by two single bonds and a double bond. The remaining valences of the carbon atoms are satisfied by four hydrogens. The structure can be represented as a cyclopropene ring.
It’s important to note that in structural isomerism, the functional groups and atoms are linked in different ways, resulting in unique arrangements of the molecules. Therefore, these isomers are assigned different IUPAC names since they may or may not contain the same functional groups. Structural isomerism highlights the versatility of carbon-based compounds and demonstrates the various possibilities of bonding arrangements within a given molecular formula.
1. Chain Isomerism
Chain isomerism, also referred to as skeletal isomerism, is a type of structural isomerism where the components of isomers exhibit different arrangements in terms of branching within the carbon chain. In chain isomers, the carbon skeleton or backbone of the molecule varies, while the molecular formula remains the same.
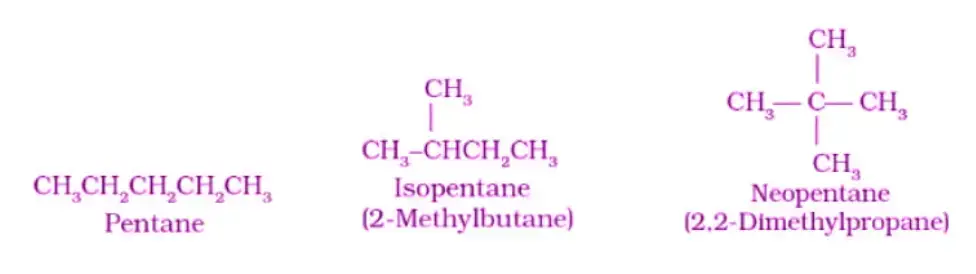
In chain isomerism, the arrangement of carbon atoms within the molecule is altered, leading to distinct structures and properties. The main point of difference between chain isomers lies in the branching pattern of carbon atoms. Let’s consider an example to better understand chain isomerism using the compound C5H12:
Example: C5H12 The molecular formula C5H12 represents a hydrocarbon with five carbon atoms and twelve hydrogen atoms. In this case, chain isomerism can be observed, as the carbon backbone of the molecule can be arranged differently, resulting in different isomers. Let’s illustrate this using condensed structural formulas:
- Pentane: Pentane is a straight-chain isomer, where the five carbon atoms are arranged in a linear fashion, forming a continuous chain. The condensed structural formula for pentane is CH3-CH2-CH2-CH2-CH3.
- 2-Methylbutane: 2-Methylbutane is a chain isomer of pentane. In this isomer, the carbon chain has a branch, with a methyl (-CH3) group attached to the second carbon atom. The condensed structural formula for 2-methylbutane is CH3-CH(CH3)-CH2-CH3.
- 2,2-Dimethylpropane: 2,2-Dimethylpropane is another chain isomer of pentane. In this isomer, the carbon chain has two branches, with methyl (-CH3) groups attached to the second and fourth carbon atoms. The condensed structural formula for 2,2-dimethylpropane is (CH3)3C-CH3.
In the example above, three chain isomers of C5H12 are shown: pentane, 2-methylbutane, and 2,2-dimethylpropane. While they all have the same molecular formula, the arrangement of carbon atoms and branching within the molecule is different for each isomer.
Chain isomerism is commonly observed in organic compounds, especially hydrocarbons, and plays a significant role in determining their physical and chemical properties. By altering the branching pattern within the carbon backbone, chain isomers can exhibit different characteristics, such as boiling points, melting points, and reactivity, despite having the same molecular formula.
2. Position Isomerism
Position isomerism is a type of structural isomerism where the positions of functional groups or substituent atoms vary within a molecule. In position isomers, the connectivity of atoms remains the same, but the location of functional groups or substituents differs, leading to distinct isomeric forms.
Position isomerism often involves the attachment of functional groups to different carbon atoms within the carbon chain of a molecule. This variation in the attachment position can significantly impact the chemical and physical properties of the isomers. Let’s consider an example to better understand position isomerism using compounds with the formula C3H7Cl:
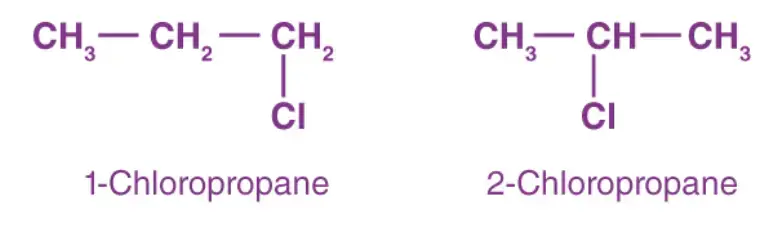
Example: C3H7Cl The molecular formula C3H7Cl represents a compound with three carbon atoms, seven hydrogen atoms, and one chlorine atom. In this case, position isomerism can be observed, as the chlorine atom is attached to different carbon atoms in the carbon chain, resulting in different isomeric structures. Let’s illustrate this using condensed structural formulas:
- 1-Chloropropane: In 1-chloropropane, the chlorine atom is attached to the first carbon atom of the three-carbon chain. The condensed structural formula for 1-chloropropane is CH3CH2CH2Cl.
- 2-Chloropropane: In 2-chloropropane, the chlorine atom is attached to the second carbon atom of the three-carbon chain. The condensed structural formula for 2-chloropropane is CH3CHClCH3.
Both 1-chloropropane and 2-chloropropane have the same molecular formula, C3H7Cl, but differ in the position of the chlorine atom within the carbon chain.
In the example above, position isomerism is evident in the compounds with the formula C3H7Cl, specifically in the different attachment positions of the chlorine atom. This variation in position can lead to variations in chemical reactivity, polarity, and other properties of the isomers.
Position isomerism is a significant concept in organic chemistry and plays a crucial role in understanding the diverse behavior of organic compounds. By changing the position of functional groups or substituents, position isomers exhibit distinct characteristics, allowing for a wide range of chemical possibilities within a given molecular formula.
3. Functional Isomerism
Functional isomerism, also known as functional group isomerism, is a type of isomerism where compounds have the same chemical formula but differ in the functional groups attached to them. In functional isomerism, the arrangement or type of functional groups within the molecule changes, leading to distinct isomeric forms.
The term “functional” refers to the specific group of atoms responsible for the characteristic chemical reactions and properties of a compound. Functional groups can include elements like oxygen, nitrogen, sulfur, and others, bonded to the carbon skeleton of the molecule. The presence or absence of different functional groups can significantly influence the behavior and reactivity of the compound.
Let’s consider an example to better understand functional isomerism using the compound C3H6O:
Example: C3H6O The molecular formula C3H6O represents a compound with three carbon atoms, six hydrogen atoms, and one oxygen atom. In this case, functional isomerism can be observed, as different functional groups can be attached to the carbon skeleton, resulting in different isomeric structures. Let’s illustrate this using condensed structural formulas:
- Propanal: Propanal is an aldehyde, where the carbon chain is attached to a carbonyl group (C=O) at the terminal carbon. The condensed structural formula for propanal is CH3CH2CHO.
- Propanone: Propanone, commonly known as acetone, is a ketone. In this isomer, the carbon chain is attached to a carbonyl group (C=O) at the central carbon. The condensed structural formula for propanone is CH3COCH3.
Both propanal and propanone have the same molecular formula, C3H6O, but differ in the type and location of the functional group (aldehyde versus ketone).
In the example above, functional isomerism is evident in the compounds with the formula C3H6O, specifically in the different functional groups (aldehyde and ketone) attached to the carbon chain.
Functional isomerism is an important concept in organic chemistry as it highlights the diverse chemical behavior and reactivity of compounds. By changing the functional groups within a molecule while keeping the same chemical formula, functional isomers exhibit distinct properties and may have different applications in various chemical processes.

4. Metamerism
Metamerism is a type of isomerism that occurs due to the presence of different alkyl chains on each side of a functional group within a molecule. It is a relatively rare form of isomerism and is typically observed in compounds containing a divalent atom, such as sulfur (S) or oxygen (O), surrounded by alkyl groups.
In metamerism, the connectivity of atoms remains the same, but the alkyl groups attached to the divalent atom differ, resulting in distinct isomeric structures. This variation in alkyl chains can significantly impact the chemical and physical properties of the compounds. Let’s consider an example to better understand metamerism using the compound C4H10O:
Example: C4H10O The molecular formula C4H10O represents a compound with four carbon atoms, ten hydrogen atoms, and one oxygen atom. In this case, metamerism can be observed, as the alkyl groups attached to the oxygen atom differ, leading to different isomeric structures. Let’s illustrate this using condensed structural formulas:
- Ethoxyethane: Ethoxyethane is a metamer of C4H10O. In this isomer, the oxygen atom is bonded to two ethyl (-C2H5) groups. The condensed structural formula for ethoxyethane is C2H5OC2H5.
- Methoxypropane: Methoxypropane is another metamer of C4H10O. In this isomer, the oxygen atom is bonded to a methyl (-CH3) group and a propyl (-C3H7) group. The condensed structural formula for methoxypropane is CH3OC3H7.
Both ethoxyethane and methoxypropane have the same molecular formula, C4H10O, but differ in the alkyl groups attached to the oxygen atom.
In the example above, metamerism is evident in the compounds with the formula C4H10O, specifically in the different alkyl chains surrounding the divalent oxygen atom.
Metamerism is a less common form of isomerism compared to other types such as structural or stereoisomerism. However, it is an important concept in organic chemistry, particularly in the study of compounds containing divalent atoms surrounded by alkyl groups. The different arrangements of alkyl chains in metamerism can result in distinct chemical reactivity and physical properties, highlighting the diversity and complexity of organic compounds.
5. Tautomerism
Tautomerism is a type of isomerism in which compounds exist in equilibrium and readily interconvert between two or more isomeric forms. These isomers differ in the position of protons and electrons within the molecule. The interconversion between tautomers occurs through an intramolecular proton transfer process.
The equilibrium between tautomers is represented by the reversible reaction:
H-X-Y=Z ⇌ X=Y-Z-H
In this equation, H, X, Y, and Z represent different atoms or groups within the molecule. The tautomeric forms coexist in equilibrium, meaning that they can convert back and forth between each other.
Keto-enol tautomerism is one of the most important examples of tautomerism. It involves the interconversion between a keto form and an enol form of a compound. The keto form typically contains a carbonyl group (C=O), while the enol form contains a hydroxyl group (-OH) connected to a carbon-carbon double bond. The interconversion between keto and enol tautomers is driven by the migration of a proton and the rearrangement of electrons.
Tautomeric transformations are often influenced by factors such as pH, temperature, and the presence of catalysts. The equilibrium between tautomers can be shifted depending on these conditions. Additionally, the relative stability and energy difference between the tautomeric forms can vary, leading to different ratios of tautomers at equilibrium.
Tautomeric phenomena are observed in various organic compounds and biochemical systems. For example, amino acids can exist as zwitterionic forms or neutral forms in solution, undergoing tautomeric interconversion. This equilibrium is crucial for the biological functions of amino acids and plays a role in their reactivity and interactions within proteins.
In summary, tautomerism involves the equilibrium interconversion between isomeric forms of compounds, where the position of protons and electrons changes. It is an important concept in organic chemistry and biochemistry, with examples like keto-enol tautomerism and amino acid tautomers. The ability of compounds to exist in different tautomeric forms contributes to their diverse chemical properties and reactivity.
6. Ring-Chain Isomerism

Ring-chain isomerism is a type of isomerism where two isomers differ in their structural arrangement, with one isomer having an open-chain structure and the other having a ring structure. This isomerism arises due to the difference in the connectivity of atoms within the molecule.
One significant distinction between the ring and chain isomers is the number of pi bonds present in each structure. Pi bonds are formed by the overlapping of p-orbitals and contribute to the double bond character between carbon atoms.
Let’s consider an example to better understand ring-chain isomerism using the compound C3H6:
Example: C3H6 The molecular formula C3H6 represents a compound with three carbon atoms and six hydrogen atoms. In this case, ring-chain isomerism can be observed as the arrangement of carbon atoms differs between the isomers.
- Propene: Propene is the open-chain isomer of C3H6. It consists of a linear arrangement of three carbon atoms with a double bond between two adjacent carbon atoms. The condensed structural formula for propene is CH2=CHCH3.
- Cyclopropane: Cyclopropane is the ring isomer of C3H6. It contains a three-membered carbon ring where each carbon atom is bonded to two hydrogen atoms. The condensed structural formula for cyclopropane is (CH2)3.
Both propene and cyclopropane have the same molecular formula, C3H6, but differ in their structural arrangement, with one being an open-chain and the other a ring structure.
In the example above, ring-chain isomerism is evident in the compounds with the formula C3H6, specifically in the arrangement of carbon atoms and the presence or absence of a ring structure.
Ring-chain isomerism is an important concept in organic chemistry, as it demonstrates how the connectivity of atoms within a molecule can influence the chemical properties and reactivity of compounds. The presence of a ring structure, as in cyclopropane, can result in different strain effects and reactivity compared to the open-chain structure, as in propene. Understanding ring-chain isomerism expands our understanding of the diverse structural possibilities and behaviors of organic compounds.
B. Stereoisomerism
Stereoisomerism refers to a type of isomerism where compounds have the same chemical formula but differ in the three-dimensional orientation of their constituent atoms. The term “stereoisomer” is often used to describe these compounds, which exhibit differences in spatial arrangements.
There are two main subtypes of stereoisomerism: conformational isomerism and configurational isomerism. Let’s explore each of them:
a. Conformational Isomerism
Conformational isomerism arises due to the rotation around single bonds, which allows different spatial arrangements of atoms within a molecule. These isomers are known as conformers or conformational isomers and exist in equilibrium, interconverting rapidly at room temperature.
A classic example of conformational isomerism is observed in cyclohexane. In its most stable conformation, known as the chair conformation, the carbon atoms alternate above and below the mean plane of the ring. Another conformation, called the boat conformation, has two opposite carbons above the plane and the other four below it. The interconversion between these conformations occurs due to the rotation of carbon-carbon bonds. However, the energy difference between these conformers is relatively low, allowing them to rapidly interconvert.
b. Configurational Isomerism
Configurational isomerism involves isomers that have distinct spatial arrangements and cannot be interconverted without breaking and reforming covalent bonds. The configurational isomers are further categorized into two subtypes: geometric isomerism (cis-trans isomerism) and optical isomerism (enantiomerism).
- Geometric Isomerism (Cis-Trans Isomerism): This type of isomerism occurs when atoms or groups are arranged differently around a rigid bond. It is commonly observed in compounds with double bonds or cyclic structures. Cis-isomers have similar groups on the same side of the molecule, while trans-isomers have them on opposite sides. For example, cis-2-butene and trans-2-butene are geometric isomers that differ in the spatial arrangement around the double bond.
- Optical Isomerism (Enantiomerism): Optical isomerism arises when molecules possess a chiral center, which is an atom bonded to four different groups. Enantiomers are non-superimposable mirror images of each other. They have the same physical and chemical properties except for their interaction with polarized light. Enantiomers are designated as “R” (rectus) or “S” (sinister) based on their configuration relative to a reference molecule. A well-known example of enantiomers is L-(+) and D-(-) forms of amino acids.
It’s important to note that conformational isomers are related to the rotation of single bonds, while configurational isomers require breaking and reforming of covalent bonds or involve chiral centers.
In conclusion, stereoisomerism encompasses the isomers that have the same chemical formula but differ in their three-dimensional orientations. Conformational isomerism involves different conformers achieved through bond rotations, while configurational isomerism includes geometric isomerism (cis-trans) and optical isomerism (enantiomerism) that result from distinct spatial arrangements and require bond breaking or chiral centers. Understanding stereoisomerism is crucial in explaining the diverse properties and behaviors of compounds in the field of chemistry.
Geometric Isomerism or Cis-trans isomerism
Geometric isomerism, also known as cis-trans isomerism, is a type of stereoisomerism that arises from the different spatial arrangements of atoms in three-dimensional space. It occurs when a molecule has restricted rotation around a bond or a rigid framework that determines the relative positions of functional groups or substituents.
The terms cis and trans are commonly used to describe the geometric isomers. Cis refers to the arrangement where similar groups or atoms are located on the same side of the molecule, while trans refers to the arrangement where similar groups or atoms are located on opposite sides.
A classic example of geometric isomerism is dichloroethene (C2H2Cl2), specifically the isomer with one chlorine atom bonded to each carbon atom. This molecule exhibits two distinct conformations, cis and trans. In the cis-isomer, the two chlorine atoms are on the same side of the double bond’s plane, while in the trans-isomer, the two chlorine atoms are on opposite sides. The cis and trans isomers are different configurations of the molecule and can exhibit different chemical and physical properties.
Geometric isomerism is not limited to organic compounds. It can also occur in inorganic coordination compounds, such as square planar complexes (MX2Y2) and octahedral complexes (MX4Y2). In these complexes, the arrangement of ligands around the central metal atom can give rise to cis and trans isomers.
In more complex organic molecules, the use of cis and trans labels can sometimes be ambiguous. The International Union of Pure and Applied Chemistry (IUPAC) recommends a more precise labeling scheme based on the Cahn-Ingold-Prelog (CIP) priority rules. These rules assign priorities to different substituents based on the atomic numbers of the atoms directly bonded to the chiral center, allowing for a more accurate description of the geometric isomerism in such molecules.
Geometric isomerism is an important concept in chemistry as it can have significant implications for the properties, reactivity, and biological activity of compounds. Understanding the spatial arrangements of atoms and the presence of geometric isomers is crucial for studying and predicting the behavior of molecules in various chemical reactions and biological processes.

Optical Isomerism or Enantiomers
Optical isomerism, also known as enantiomerism, is a form of stereoisomerism exhibited by compounds that have similar bonds but different spatial arrangements of atoms, resulting in non-superimposable mirror images. Enantiomers are pairs of molecules that are mirror images of each other and cannot be aligned through rotations or translations alone. They are often referred to as “left-handed” and “right-handed” forms.
A distinguishing feature of enantiomers is their ability to rotate the plane of polarized light. Dextro enantiomers, also known as (+)-enantiomers, rotate the plane of polarized light to the right, while laevo enantiomers, also known as (-)-enantiomers, rotate it to the left.
One classic example of enantiomers is bromochlorofluoromethane (CHFClBr). The two enantiomers of this compound can be differentiated based on the direction of the path from F to Cl to Br as seen from the hydrogen atom. Converting one enantiomer into the other would require severe strain or bond breaking. The energy barrier between the two enantiomers is typically high, resulting in minimal interconversion at room temperature.
In contrast, compounds like chlorofluoromethane (CH2ClF) are not chiral because their mirror image can be obtained through a half-turn rotation around a suitable axis.
Axial isomerism is another type of isomerism related to enantiomers. An example is 2,3-pentadiene, a hydrocarbon with two overlapping double bonds. Although the molecule has an axis of symmetry, its mirror image is not superimposable. This type of isomerism is known as axial isomerism.
Enantiomers generally exhibit identical behavior in chemical reactions, except when interacting with other chiral compounds or in the presence of chiral catalysts, such as enzymes. Due to these interactions, enantiomers often have different effects and roles in biological systems. In biochemistry and food science, enantiomers of chiral molecules like glucose are considered distinct substances with varying properties.
Each enantiomer of a chiral compound has the same magnitude but opposite sense of rotation on polarized light passing through it. This optical rotation can be used to distinguish and measure the concentration of enantiomers in a solution. The term “optical isomers” was commonly used to refer to enantiomers because of this phenomenon, but it is now discouraged by the International Union of Pure and Applied Chemistry (IUPAC) due to its ambiguity.
Stereoisomers that are not enantiomers are called diastereomers. Some diastereomers may contain a chiral center, while others may not. Enantiomers and diastereomers represent different configurations of molecules and can have distinct properties and roles in various chemical and biological processes.

Ionization Isomerism
Ionization isomerism refers to a type of isomerism where a compound, despite having the same composition, gives rise to different ions when dissolved in a solution. This phenomenon is known as ionization isomerism. It occurs when the counter ion in a complex salt can act as a potential ligand and displace another ligand, which then becomes the counter ion.
An illustrative example of ionization isomerism involves the compounds [Co(NH3)5SO4]Br and [Co(NH3)5Br]SO4. These two compounds exhibit ionization isomerism because they generate different ions in solution despite having the same overall composition. The position of the counter ion and its ability to exchange with other ligands result in the formation of different ions.
Ionization isomers can be prepared using specific methods. For instance:
- [CoBr(NH3)5]SO4 can be prepared by dissociating as follows: [CoBr(NH3)5]SO4 → [CoBr(NH3)5]2+ + SO42− (Red-Violet color)
- [CoSO4(NH3)5]Br can be prepared by dissociating as follows: [CoSO4(NH3)5]Br → [CoSO42−(NH3)5]+ + Br− (Red color)
These examples demonstrate how the exchange of ligands and counter ions within a complex salt can lead to the formation of ionization isomers with different ion compositions.
In summary, ionization isomerism occurs when a compound produces different ions in a solution while maintaining the same overall composition. This type of isomerism arises due to the ability of the counter ion to displace other ligands, resulting in the formation of distinct ions. The study of ionization isomerism provides insights into the behavior of complex salts and their ionization properties.
What is Topoisomers?
- Topoisomers refer to isomers of large molecules that differ in their overall topology or arrangement in space, even when there is no specific geometric constraint that separates them. These isomers arise due to variations in the way long chains can be twisted or knotted, leading to distinct topological forms. The interconversion between these topoisomers is often hindered by bulky substituents or the closure of cycles, as observed in circular DNA and RNA plasmids.
- One interesting aspect of topoisomers is the presence of mirror-image enantiomer pairs among certain knots. These mirror-image forms are considered topological isomers or topoisomers. The topological differences in these isomers arise from their unique arrangements in three-dimensional space.
- Furthermore, it is possible for two or more molecules to be linked together in a catenane through topological linkages, even in the absence of chemical bonding between them. In the case of large molecules, such as catenanes, the linking can occur in multiple topologically distinct ways, resulting in different topoisomers. This topological isomerism in catenanes adds another layer of complexity to their structure and behavior.
- Another type of topological isomerism involves cage compounds, such as helium enclosed in dodecahedrane (He@C20H20) and carbon peapods. These compounds possess large internal voids with restricted or no openings, leading to distinct topological arrangements. The presence of these voids and their encapsulated content gives rise to different topoisomers.
- In summary, topoisomers represent isomers of large molecules that exhibit variations in their overall topology or arrangement in space. These isomers can arise from twisted or knotted chains, mirror-image enantiomer pairs in knots, topological linkages in catenanes, or the presence of internal voids in cage compounds. The study of topoisomers contributes to a deeper understanding of the diverse structures and properties that can emerge in large molecules.
Isotopes and spin
Isotopes and spin can give rise to different forms of isomerism in molecules. Isotopomers are isomers that result from the replacement of one or more atoms by their isotopes, creating structural and/or stereoisomers within a molecule or ion. For example, substituting deuterium (D) for hydrogen (H) in an ethane molecule yields two distinct structural isomers: 1,1-dideuteroethane (HD2C-CH3) and 1,2-dideuteroethane (DH2C-CDH2). These isotopomers have different properties and do not easily interconvert. Similarly, substituting deuterium for hydrogen in chlorofluoromethane (CH2ClF) generates a pair of chiral enantiomers, CHDClF, distinguished by their optical activity.
Isotopomers are different from isotopologs, which are molecules that have the same structure but differ in their isotopic composition. For example, C2H5D and C2H4D2 are isotopologues but not isotopomers, as they do not exhibit structural or stereoisomeric differences when isotopes are replaced.
Spin isomerism is another type of isomerism that arises from differences in the relative spin magnetic quantum numbers (ms) of atomic nuclei within molecules. Molecular hydrogen provides an example of spin isomerism. It can exist in two long-lived states known as spin isomers or nuclear spin isomers: parahydrogen and orthohydrogen. Parahydrogen has the spins of its two nuclei pointing in opposite directions, while orthohydrogen has the spins pointing in the same direction.
In summary, isotopomers are isomers formed by replacing atoms with their isotopes, creating distinct structural and/or stereoisomers. Spin isomerism arises from differences in the spin states of atomic nuclei within molecules. Understanding these forms of isomerism provides insights into the behavior and properties of isotopic variants and nuclear spin states in molecules.
What is Isomerization?
- Isomerization refers to the process in which a molecule undergoes a transformation, resulting in another molecule with the same atoms but rearranged in a different configuration. This rearrangement can occur spontaneously under certain conditions. In cases where isomerization takes place within the same molecule, it is known as a rearrangement reaction.
- Isomerization is often observed when isomers have comparable bond energies, allowing them to exist in roughly equal amounts if they can freely interconvert. The interconversion between isomers occurs when the energy barrier between them is not too high. This phenomenon is particularly relevant in cases of intramolecular isomerization.
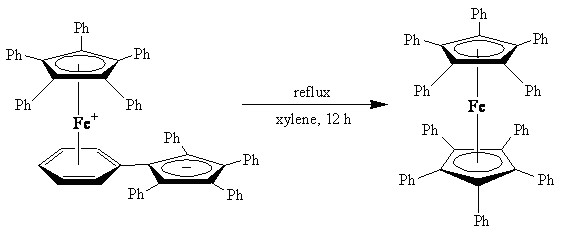
- One example of isomerization is found in organometallic compounds, such as the production of decaphenylferrocene, [(η5-C5Ph5)2Fe], from its linkage isomer. This process involves a rearrangement of the phenyl groups bonded to the ferrocene core.
- Isomerization reactions are also prevalent in industrial synthesis. For instance, the production of fumaric acid involves the cis-trans isomerization of maleic acid. By converting maleic acid into its isomer fumaric acid, the desired compound can be obtained.
- In addition to organic chemistry, isomerization plays a role in molecular biology. Topoisomerases are enzymes that facilitate changes in the topology of circular DNA molecules by cutting and rejoining the DNA strands. Through this process, topoisomerases can alter the arrangement of DNA, providing essential mechanisms for DNA replication, repair, and transcription.
- In summary, isomerization is a process in which molecules undergo rearrangement, resulting in a different configuration while maintaining the same atoms. It can occur spontaneously or through intramolecular rearrangement reactions. Isomerization reactions have diverse applications in various fields, including organic synthesis, industrial processes, and molecular biology.

What is Resonance forms?
- Resonance forms are multiple representations used to describe the delocalized bonding in certain molecules or ions. They are not actual distinct compounds but serve as a way to depict the electron distribution within the molecule.
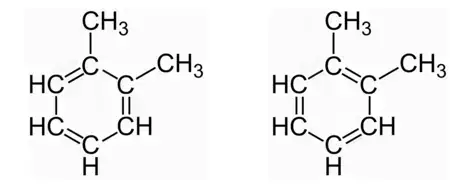
- A classic example of resonance forms is observed in 1,2-dimethylbenzene, commonly known as o-xylene. This molecule is often represented by two apparent structural isomers, as shown in the figures. However, neither of these structures accurately represents the true nature of o-xylene.
- In reality, o-xylene exists as a single isomer with a benzene core and two methyl groups in adjacent positions. The concept of resonance is employed to describe the delocalization of pi electrons within the molecule. The true structure of o-xylene cannot be represented by a single Lewis structure because the pi electrons are shared over the entire molecule rather than being localized between specific carbon-carbon bonds.
- The resonance forms of o-xylene demonstrate that the bonding electrons are distributed across all carbon-carbon bonds, resulting in a more stable and delocalized system. The actual structure of o-xylene is a hybrid or combination of these resonance forms, with the electron density being shared among the different positions.
- Resonance forms are indicated by using curved arrows to show the movement of electrons between different positions in the molecule. These arrows illustrate the shifting of electron density and help visualize the delocalization of electrons.
- The concept of resonance is not limited to o-xylene but applies to other molecules as well. It is commonly observed in molecules with conjugated systems, such as benzene and other aromatic compounds, where pi electrons are delocalized over a larger region.
- Resonance forms are a useful tool in understanding the electronic structure and chemical behavior of molecules. They provide a more accurate representation of the actual bonding and electron distribution in systems that exhibit delocalization.
What is isomerization, isomerase, isomeric, isomerism?
- Isomerization: Isomerization is a chemical process in which one isomer is transformed into another isomer. It involves the rearrangement of atoms within a molecule, resulting in a different structural or spatial arrangement while maintaining the same composition.
- Isomerase: An isomerase is an enzyme that catalyzes the isomerization of a molecule. It facilitates the conversion of one isomer into another by rearranging the atoms within the molecule. Isomerases play crucial roles in various biological processes, including metabolism and synthesis of important molecules.
- Isomeric: Isomeric refers to the characteristic of having or exhibiting isomers. It describes a set of compounds or molecules that have the same chemical formula but differ in their structural or spatial arrangement, resulting in distinct properties and characteristics.
- Isomerism: Isomerism is a phenomenon where two or more compounds have the same molecular formula but differ in their structural or spatial arrangement, leading to distinct chemical and physical properties. Isomerism can arise due to differences in connectivity (structural isomerism), spatial orientation (stereoisomerism), or both.
Isomerism can be further classified into various types, including constitutional isomerism (differing connectivity), stereoisomerism (differing spatial arrangement), geometric isomerism (cis-trans isomerism), optical isomerism (enantiomers), and others.
Isomerism is a fundamental concept in chemistry and has significant implications in understanding the behavior, reactivity, and properties of different chemical compounds.
What is phosphoglucose isomerase?
- Phosphoglucose isomerase (PGI), also known as glucose-6-phosphate isomerase (GPI), is an enzyme that catalyzes the interconversion of glucose-6-phosphate (G6P) and fructose-6-phosphate (F6P) in the glycolysis pathway. It is an essential enzyme in glucose metabolism and is found in various organisms, including bacteria, plants, and animals.
- The reaction catalyzed by phosphoglucose isomerase involves the conversion of an aldose (glucose-6-phosphate) to a ketose (fructose-6-phosphate). Specifically, it rearranges the carbonyl group and the hydroxyl groups on carbon 1 and carbon 2, resulting in the isomerization of the glucose-6-phosphate molecule to fructose-6-phosphate.
- The role of phosphoglucose isomerase in glycolysis is to facilitate the subsequent metabolism of glucose by converting it to a form (fructose-6-phosphate) that can continue through the pathway. It plays a crucial role in energy production and the generation of intermediates necessary for various cellular processes.
- Phosphoglucose isomerase has been widely studied due to its importance in glycolysis and its association with certain diseases. Mutations in the gene encoding phosphoglucose isomerase can lead to a rare genetic disorder known as phosphoglucose isomerase deficiency, which is characterized by impaired energy metabolism and various clinical manifestations.
- In addition to its role in glycolysis, phosphoglucose isomerase has also been found to have non-glycolytic functions, such as participating in immune responses, cell signaling, and tumor progression. Its diverse functions highlight the significance of this enzyme beyond its involvement in glucose metabolism.
What is Ribose 5 phosphate isomerase deficiency?
Ribose 5-phosphate isomerase deficiency, also known as RPI deficiency or RPIA deficiency, is a rare inherited metabolic disorder that affects the pentose phosphate pathway (PPP). The PPP is a series of biochemical reactions that occur in cells and is responsible for the production of ribose-5-phosphate, a crucial molecule for nucleotide synthesis and the production of energy in the form of ATP.
In individuals with RPI deficiency, there is a mutation in the gene that encodes for the enzyme ribose 5-phosphate isomerase (RPI). This enzyme is responsible for converting ribose-5-phosphate into ribulose-5-phosphate, an essential step in the PPP. The deficiency of RPI leads to a disruption in the normal functioning of the pathway, resulting in a decrease in the production of ribose-5-phosphate and its downstream products.
The symptoms and severity of RPI deficiency can vary among affected individuals. Some common clinical features may include intellectual disability, growth retardation, microcephaly (small head size), craniofacial abnormalities, skeletal abnormalities, and other physical and developmental abnormalities. The condition may also lead to abnormalities in red blood cells, resulting in anemia.
RPI deficiency is typically inherited in an autosomal recessive manner, meaning that an affected individual has inherited two copies of the mutated gene (one from each parent). Genetic testing is required to confirm the diagnosis of RPI deficiency. Treatment options for this condition are currently limited, and management primarily involves supportive care and symptomatic treatment.
It’s important to note that while I strive to provide accurate and up-to-date information, the field of medicine is constantly evolving, and new discoveries may emerge regarding Ribose 5-phosphate isomerase deficiency. It is always recommended to consult with a healthcare professional or geneticist for specific concerns or questions related to this condition.
What is linkage isomerism?
Linkage isomerism is a type of structural isomerism that occurs in coordination compounds. Coordination compounds are molecules or ions in which a central metal atom or ion is surrounded by ligands (atoms, ions, or molecules) that are coordinated to the metal through coordinate covalent bonds.
In linkage isomerism, the coordination compound can exist in two or more isomeric forms that differ in the way the ligand is attached to the metal center. The isomers arise from the possibility of ligands binding to the metal through different atoms or groups within the ligand molecule.
To illustrate linkage isomerism, let’s consider an example using the complex ion [Co(NH3)5NO2]2+. In this compound, the cobalt ion (Co2+) is coordinated to five ammonia ligands (NH3) and one nitrite ligand (NO2-).
Now, in one isomeric form, the nitrite ligand can bind to the cobalt ion through the nitrogen atom (N) of the nitrite group, resulting in the coordination compound [Co(NH3)5N(=O)O]+. This is called the nitrito isomer.
In another isomeric form, the nitrite ligand can bind to the cobalt ion through the oxygen atom (O) of the nitrite group, resulting in the coordination compound [Co(NH3)5O(=N)O]+. This is called the nitro isomer.
The key difference between the nitrito and nitro isomers is the location of the coordination bond between the cobalt ion and the nitrite ligand.
Linkage isomerism can also occur with other ligands that have the potential to coordinate through different atoms or groups. For example, ligands like SCN- (thiocyanate) and NCS- (isothiocyanate) can exhibit linkage isomerism.
It’s important to note that linkage isomerism is specific to coordination compounds, and the isomers are distinguished by the arrangement of ligands around the central metal atom or ion.
What is Saccharide isomerate?
“Saccharide isomerate” typically refers to a complex mixture of isomeric forms of saccharides, particularly monosaccharides or simple sugars. Isomers are compounds that have the same chemical formula but differ in the arrangement or spatial orientation of their atoms. In the case of saccharides, isomers can vary in the arrangement of hydroxyl groups (OH) around the carbon atoms.
Monosaccharides, such as glucose, fructose, and galactose, are fundamental building blocks of carbohydrates and serve as important energy sources in living organisms. These monosaccharides can exist in different isomeric forms, including structural isomers (aldoses and ketoses) and stereoisomers (such as enantiomers and diastereomers).
When a mixture of different saccharide isomers is referred to as “saccharide isomerate,” it implies that the mixture contains various forms of saccharides, which may have different properties, such as sweetness, solubility, and reactivity. The isomerate may be obtained from natural sources, such as fruits or plant extracts, or it can be produced through chemical or enzymatic processes.
Saccharide isomerates can have applications in the food industry, where they may be used as sweeteners or flavor enhancers. The mixture of different isomeric forms can provide a more complex and balanced taste profile compared to individual sugars. Additionally, these isomerates can exhibit different functional properties, such as better solubility or stability, depending on the specific isomers present.
It’s important to note that the composition and properties of saccharide isomerates can vary depending on the specific mixture and the methods used for their production. Therefore, it is recommended to refer to specific products or formulations to understand the exact composition and characteristics of a particular saccharide isomerate.
Importance of Isomerism
Isomerism, including optical isomerism, holds significant importance in various fields, particularly in chemistry and pharmaceuticals. Here are some key points highlighting the importance of isomerism:
- Distinctive Properties: Isomers of a molecule can exhibit different physical and chemical properties, such as boiling point, melting point, solubility, reactivity, and biological activity. This provides a deeper understanding of the structure-function relationship and enables scientists to tailor compounds with specific properties.
- Drug Development: Isomerism plays a crucial role in drug development and testing. Enantiomers, which are optical isomers, can have different pharmacological effects in the human body. Thalidomide, as a tragic example, demonstrated the significance of testing all optical isomers of drugs for safety and efficacy. Rigorous testing ensures that only the desired enantiomer is used in pharmaceutical formulations.
- Aroma and Flavor: Isomers can have distinct smells and tastes. This property is often observed in organic compounds, such as carvone. The (R)-carvone is responsible for the minty aroma in leaves, while the (S)-carvone is responsible for the distinct smell in caraway seeds. Understanding the isomeric composition helps in the identification and characterization of aromatic compounds.
- Coordination Chemistry: Isomerism in coordination compounds influences their structural arrangements and properties. Linkage isomerism, as discussed earlier, impacts the connectivity of ligands to the central metal ion, leading to variations in color, stability, and reactivity. This knowledge is valuable for designing catalysts and understanding complex chemical reactions.
- Material Science: Isomerism plays a role in the development of new materials with tailored properties. Isomers can exhibit different crystal structures, electronic configurations, and optical properties. By controlling the isomeric composition, scientists can manipulate the physical properties of materials, enabling advancements in electronics, optics, and energy storage.
- Stereochemistry: Isomerism is essential in stereochemistry, which focuses on the three-dimensional arrangement of atoms within molecules. Stereoisomers, including geometric isomers and optical isomers, have distinct spatial arrangements that affect their interactions with other molecules, including enzymes and receptors. Understanding stereochemistry aids in drug design, bioactive molecule synthesis, and molecular recognition processes.
In summary, isomerism is of utmost importance in various scientific disciplines. It helps elucidate the relationship between structure and properties, aids in drug development and safety testing, contributes to the understanding of aroma and flavor compounds, influences the behavior of coordination compounds, enables the design of new materials, and plays a pivotal role in stereochemistry and molecular recognition. The study and recognition of different isomeric forms are crucial for advancing scientific knowledge and applications across multiple fields.
FAQ
What is isomerism?
Isomerism is a phenomenon in chemistry where compounds with the same molecular formula have different structural arrangements or spatial orientations, resulting in distinct chemical and physical properties.
What causes isomerism?
Isomerism is caused by the different ways atoms can be connected or arranged within a molecule, leading to variations in bond connectivity, spatial orientation, or overall structure.
How many types of isomerism are there?
There are several types of isomerism, including structural isomerism, stereoisomerism (which includes geometric and optical isomerism), tautomeric isomerism, ionization isomerism, and others.
What is structural isomerism?
Structural isomerism occurs when compounds have the same molecular formula but differ in the arrangement of their atoms, such as chain isomerism, position isomerism, and functional group isomerism.
What is stereoisomerism?
Stereoisomerism refers to the isomerism arising from the different spatial arrangement of atoms in a molecule. It includes geometric isomerism (cis-trans isomerism) and optical isomerism (enantiomerism).
What is geometric isomerism?
Geometric isomerism occurs when compounds have the same molecular formula and bond connectivity but differ in the arrangement of substituents around a rigid bond, resulting in cis and trans isomers.
What is optical isomerism?
Optical isomerism, also known as enantiomerism, arises when compounds have the same molecular formula and connectivity but are non-superimposable mirror images of each other, leading to differences in optical activity.
What is tautomeric isomerism?
Tautomeric isomerism involves the interconversion of compounds through the migration of atoms or groups within the molecule, leading to different structural arrangements and chemical properties.
What is ionization isomerism?
Ionization isomerism occurs when compounds exhibit different ionization behavior in solution, despite having the same composition. It arises from the interchange of ligands and counter ions in a complex.
Why is isomerism important in chemistry?
Isomerism is important because it highlights the significance of structural and spatial arrangements in determining the properties and behavior of chemical compounds. It helps explain the diversity and complexity of organic and inorganic molecules and plays a crucial role in fields such as drug design, materials science, and biochemistry.
So how can I save it on PDF reader
Isomerism in complex ions.