Ion exchange chromatography (IEC) is a chromatographic method that exploits the charge properties of the molecules. It employs an insoluble matrix, which is covalently bonded with a charged group, as a stationary phase. These groups will then also have the ability to attract and bind ions that have the opposite charge and that are in the mobile phase, which is usually an aqueous solution. The gist of IEC is the reversible transfer of ions between ions in the sample and those fixed to the mass of the stationary phase. Various factors influence the interaction strength and therefore the retention time of each ion on the column, such as the charge and size of the ions, the strength of the ionic interactions, and mobile phase conditions (pH and ionic strength). IEC is used in a broad range of analytical and preparative processes, including the purification of proteins, peptides, nucleic acids, and other charged biomolecules, along with the resolution of inorganic ions for water treatment, environmental analysis, and other industrial processes. Due to its high specificity, resolution and ability to separate complex mixtures, the technique is a well-established tool for biochemistry, molecular biology and analytical chemistry.
Ion exchange mechanism
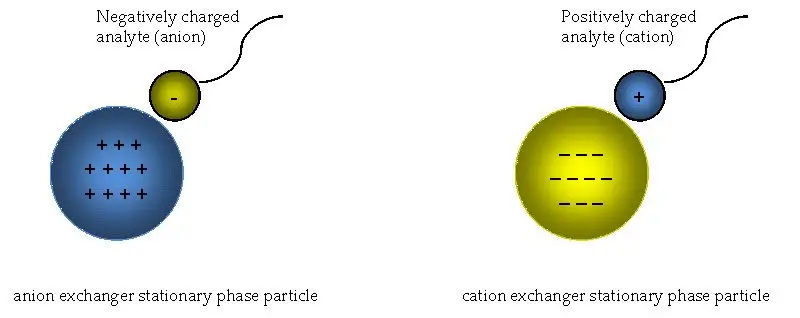
You have a sample mixture of charged molecules (i.e. proteins, salts) that contain both positive and negative charges, and you want to separate them based on charge. Use ion exchange chromatography. Essentially, ion exchange chromatography uses a charged solid stationary phase – charged resin beads like a magnet – with many charged surfaces – and a buffered liquid mobile phase.
The liquid buffer is where the sample sits. The stationary phase consists of small charged fractions; either positive ions (i.e. sodium ions) or negative ions (i.e. chloride ions), while the buffer contains everything else. Once the sample is injected into the system, the charged components interact with each other in opposite charge and exchange. For example, if the stationary phase is made of negatively charged resin beads, some of the positively charged components of the sample will bind to the negatively charged fractions and release some of the negatively charged ionic species previously bound in the buffer. It’s an ion game.
Your molecules are competing, and their charged binding sites determine how well they stay attached. If your molecule is positive and someone else’s is attached to a negatively charged site, less positively charged than yours, yours knocks the other one out of the site. Yet you can generate some of this separation with the pH of the buffer, the salt.
For example, by increasing the salt concentration to just four percent saturation, the cationic binding sites that were saturating them gave way, effectively removing your molecules and allowing you to remove them one fraction at a time. It’s like too much salt. It’s like seasoning something. Things fall apart. It’s not finicky. It uses everything from simple salts to universal proteins. This means that if you have positively charged fractions, you use a negatively charged stationary phase (cation exchange) and vice versa for the negatively charged ones.
Even the pH of the buffer is important because it is associated with the charge of the fractions. A protein sticks at one pH but passes through at another. These days, it is easier to control this phenomenon because separating these elements that take longer takes longer thanks to technology. In the end, it is all about winning and bonding.
The molecules stick together and after a while they break off and are carried away by the waters, which gives the most heterogeneous solutions the opportunity to come and go. Whether it’s formulating the ideal pure version of a medicine to help the world or assessing the level of impurities in a glass of water, the fact that this phenomenon can occur anywhere in the world and is done with such precision sets it above any other phenomenon in any laboratory.
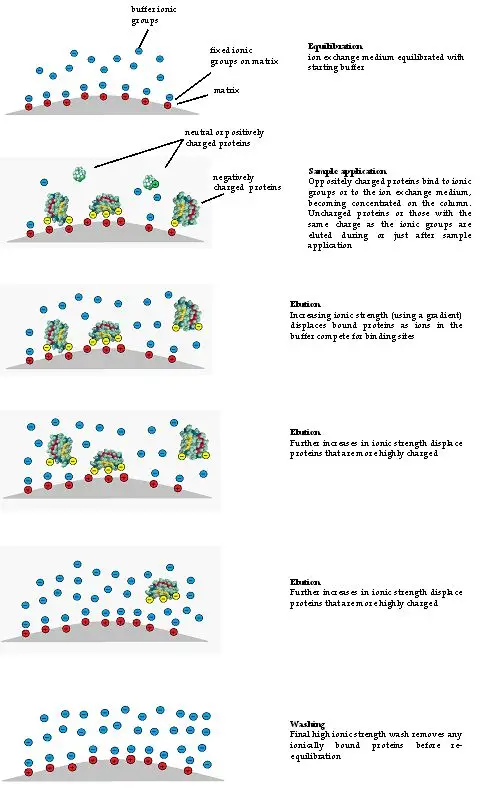
Stationary phase of Ion Exchange Chromatography
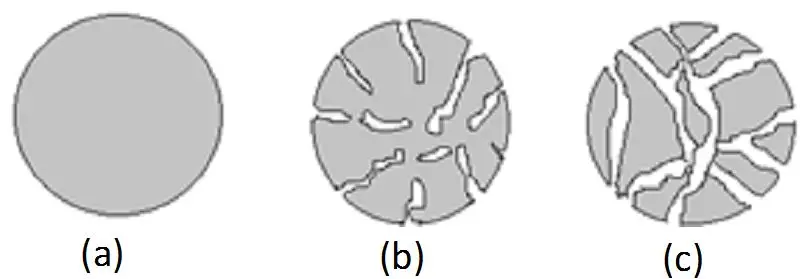
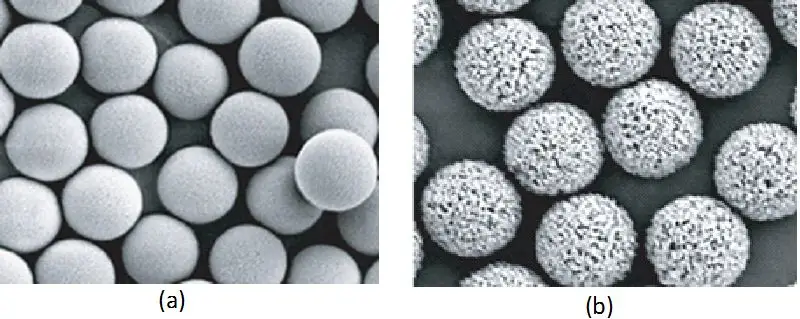
When diving into ion exchange chromatography, the stationary phase is where the magic happens. Think of it like a specialized filter that grabs onto molecules based on their charge. But it’s not just any filter—it’s a carefully designed system with a lot of moving parts. Let’s break it down in plain terms, the way you’d explain it to someone over coffee.
Picking the Right Setup
First off, choosing the right stationary phase isn’t a one-size-fits-all situation. You’ve got to consider what you’re working with. Is your sample full of positively charged molecules? Then a cation exchanger (loaded with negative groups) is your go-to. If you’re dealing with negatively charged stuff, an anion exchanger (positive groups) makes more sense. But it’s not just about charge—you also need to think about how fast you want things to flow through the column or how “sticky” the matrix might be to your sample. Mess this up, and your separation could turn into a messy guessing game.
What’s It Made Of?
The backbone of the stationary phase is usually something inert, like a plastic or polymer, but it’s jazzed up with charged groups that do the heavy lifting. These groups are like little magnets—say, sulfonic acid groups for strong cation exchangers or quaternary amines for anion ones. The trick is that these groups aren’t permanent; they can swap out their counterions (like sodium or chloride) for your target molecules. Imagine a parking garage where some cars get kicked out so others can take their spot—that’s basically how the exchange works.
Matrix Matters
The physical structure of the matrix is a big deal. Some are spongy and porous (great for big molecules like proteins), while others are tight and dense (better for small ions). Materials like agarose or cellulose are common because they’re stable and don’t react with much, but synthetic options (like polystyrene) are tougher for harsh conditions. Ever heard of dextran or polyacrylamide? Those are popular too, each with their own quirks. For example, agarose swells in water, which can slow things down, while synthetic resins stay rigid for faster flow rates.
Porosity: Size Does Matter
If your molecules are bulky, you’ll want a macroporous matrix—it’s like having wide highways instead of narrow alleys. More pores mean more surface area for binding, which boosts capacity. But if you’re separating tiny ions, a non-porous or microporous setup might give sharper results. It’s all about matching the matrix’s “personality” to your sample’s needs.
Capacity and Chemistry
The ion exchange capacity tells you how much the matrix can hold. More functional groups mean higher capacity, but it’s a balancing act—too many groups can lead to nonspecific binding. pH also plays a role here. For instance, a strong cation exchanger keeps its charge even at low pH, while weak exchangers might lose their grip if conditions change. You don’t want your stationary phase crumbling or losing its charge mid-experiment, so chemical stability across different pH levels and solvents is non-negotiable.
Flow and Resolution
Ever wonder why some separations are crisp while others look blurry? A lot comes down to particle size. Smaller, uniform particles (like perfectly graded sand) create tighter pathways, leading to better resolution—but they also slow the flow. Larger particles let things move faster but might miss finer details. It’s like choosing between a fine-tipped pen and a marker: one gives precision, the other speed.
Why This All Matters
At the end of the day, the stationary phase is the unsung hero of ion exchange chromatography. Whether you’re purifying proteins, analyzing pharmaceuticals, or cleaning up environmental samples, the right combination of matrix, functional groups, and physical properties can make or break your results. Skimp on understanding these details, and you’ll spend hours troubleshooting. Nail it, though, and you’ll get clean separations that feel almost effortless.
Mobile phase (Eluent)
Alright, let’s talk about the unsung hero of ion exchange chromatography: the mobile phase, or as the lab folks call it, the eluent. You might not give it much thought, but this liquid is basically the Uber driver for your molecules, shuttling them through the column while making sure everything gets sorted neatly. Here’s the lowdown on why it’s such a big deal—no jargon overload, promise.
First off, what’s in this stuff? Usually, it’s water jazzed up with salts like sodium chloride (yes, the same stuff you sprinkle on fries) or other ionic buddies. Sometimes they’ll toss in a splash of organic solvent—think of it as a dash of vodka in a cocktail—to help dissolve stubborn compounds. But don’t get too excited; we’re talking lab-grade here, not happy hour.
Now, why NaCl? Turns out, it’s gentle on proteins. Imagine handling a delicate soufflé; you don’t want to collapse it. Same logic. But here’s the kicker: not all salts play nice. Some are like bull in a china shop, messing with retention times or muddying your peaks. Anion exchangers, for instance, can be picky about their salt partners.
pH is another biggie. If your eluent’s pH is off, your proteins might throw a fit—denaturing, aggregating, the whole drama. Buffers swoop in to save the day here, keeping the pH steady so your molecules stay chill. And let’s not forget those competing ions. They’re like bouncers at a club, nudging your analytes off the column when it’s time to leave. More ions? Faster elution. Simple math.
Flow rate and temperature matter more than you’d think. Crank up the flow, and you’ll get results faster, but rush job might mean sloppy separation. Heat things up, though, and resolution can improve—like how warm syrup flows smoother than cold. Just don’t overdo it; nobody likes a denatured protein smoothie.
Oh, and bubbles? Total party poopers. Degassing the eluent isn’t just busywork. Air pockets can wreck your column’s flow, and dissolved CO2? That’s a sneaky pH saboteur. Pro tip: always degas. Your future self will thank you.
Before you even inject that sample, equilibration is key. It’s like preheating the oven—skip it, and your results will be as inconsistent as a half-baked cake. Once everything’s stable, you’ve got two choices: isocratic elution (steady as she goes) or gradient (ramp up the ionic strength like a DJ turning up the bass). Each has its vibe, depending on how stubborn your analytes are.
And hey, don’t sleep on additives. These are the unsung wingmen—stuff like stabilizers or detergents that keep your proteins from falling apart mid-run. Think of them as molecular bodyguards.
So there you have it. The eluent isn’t just “water with salt.” It’s a finely tuned cocktail of chemistry, physics, and a dash of finesse. Mix it right, and your chromatography sings. Mess it up? Well, let’s just say the results won’t be pretty. But hey, that’s science for you—equal parts art and precision.
Buffer
In the context of ion exchange chromatography, a buffer (eluent, mobile phase component) stabilizes pH throughout the separation process; when pH is stabilized, the charge of the stationary phase and analytes remain unchanged, and successful separation occurs.
- Specifically: Why control pH? A buffer is used to control the pH of the mobile phase during ion exchange chromatography to ensure a consistent charge is retained during pH fluctuations.
- Common buffers: aqueous salts or combinations of salts or low percent organic solvents; for example, sodium chloride (NaCl) is a common buffer because it minimally affects protein tertiary structure.
- Factors in choosing a buffer: Buffers are selected based on charge, strength, pH, and stationary phase/sample material compatibility.
- Factors making a buffer ideal: Buffers should be highly buffered relative to operating pH, solubility, purity, and cost. They should not be detrimental to conductivity and should not react adversely with the stationary phase or other buffers.
- Buffer-Ion Exchanger Interaction: The buffer used cannot interact with the ion exchange resins. The typical buffers are acetate, phosphate, and Tris. Acetate has pH fluctuations away from expected, so this must be noted and conditions assessed. Phosphates will precipitate with divalent ions; therefore, ensure that your samples are not contaminated with Ca2+, Mg2+, etc. Tris has the greatest incidence of interaction with the greatest level of such resins; therefore, it should be avoided. One needs a buffer that does not react with the resin to acquire accurate data.
- Buffer Additives and Concentrations Matter: Buffers need to be buffered at proper concentrations. For example, with beta-hydroxylamine, an overabundance of buffer was used—4 times the amount should not be as this could adjust its buffering capability. Also, methanol can wash ion-exchanged columns, but it should NOT be in the buffers. Finally, NaCl, KCl, and MgCl2 are non-buffering salts which may be in the make-up of the buffer constitution, and it’s fine; however, it’s seldom necessary. Buffering agents could be included to guarantee protein stabilization so it won’t precipitate or to prevent degradation by enzymatic action. Yet, these stabilizing characteristics must be determined that do not interfere with the ion exchange.
Detection
It is important to detect eluted eluates for identifying and quantifying sample components in ion exchange chromatography. Different detection methods are used, each with their own applications and advantages:
- Conductivity Detection:The conductivity detector, the most commonly used detector for ion exchange chromatography, measures the conductance of solute ions within the eluent. This approach makes conductivity detection highly sensitive, particularly for solutes with high conductance, and an eluent having low background conductance. Low ion exchange capacity columns and dilute elution are preferred for optimal sensitivity.
- UV Detection: Their use extends to ion exchange chromatography, especially for the detection of anions. For anions that exhibit weak to non-existent absorbance in the UV region, a strongly absorbing eluent anion can be chosen that increases the efficiency of this spectrophotometric detection.
- Eluent Anion (E-): Choice of eluent anion is important, especially with UV detection. For example, anions that contain a benzene ring are appropriate because they can exhibit good absorbance in the UV range. The eluent anion must be compatible with the chosen detection method. The eluent anion must either possess much lower conductivity than the ions in the sample, or it must be a form that can be chemically suppressed to a non-ionic form using a chemical suppression system for conductivity detection.
- Data Collection and Analysis:In modern times, after completing an ion exchange chromatography, results are usually saved digitally: Computers log retention times and peak areas. The retention times are important in identifying unknown peaks by comparison with known standards, while the peak areas can be compared with standards at known concentration to determine the concentration of the analyte.
- Detection Method Compatibility:While choosing elution anion for its detection by spectrophotometry, its absorption in the UV or visible regions is often the most important criteria. The type of anion in the eluent and its concentration vary based on the properties of the ion exchanger and the types of anions being separated.
References
- Bahadir, O. (2013). Ion-Exchange Chromatography and Its Applications. InTech. doi: 10.5772/55744