The iodine test is a simple chemical test that is used to detect the presence of carbohydrate mainly starch. It is the process in which iodine solution (iodine dissolved in potassium iodide) is added to the given sample. If starch is present in the sample the colour of the solution changes from yellow-brown to blue-black. This colour change occurs because iodine molecules get trapped inside the helical structure of amylose present in starch forming a starch–iodine complex. This test is specific for starch and does not give positive result with simple sugars like glucose or sucrose. The reaction is temperature sensitive as heating breaks the helical structure and the blue colour disappears but reappears again on cooling.
Objectives of Iodine Test
- To detect the existence of polysaccharides, primarily starch.
Principle of Iodine Test
The principle of iodine test is based on the interaction between iodine and starch molecule. Iodine is not soluble in water therefore it is dissolved in potassium iodide to form iodine solution. Starch mainly contains amylose which is present in a helical form. This helical structure has a hollow space inside which allows iodine molecules to enter and form a starch–iodine complex. As a result of this complex formation the colour of the solution changes from yellow-brown to blue-black. This reaction is specific for starch as simple carbohydrates do not have helical structure and hence do not show colour change. The process is reversible in nature because on heating the helix is destroyed and colour disappears while on cooling the helix is again formed and blue-black colour reappears.
Requirements
The requirements for iodine test are as follows–
- Chemical reagents–
- Iodine solution (iodine dissolved in potassium iodide)
- Distilled water
- Ethanol (required in case of leaf test to remove chlorophyll)
- Laboratory apparatus–
- Heating and preparation tools–
- Water bath
- Hot plate or Bunsen burner
- Knife or scalpel (for solid samples)
- Mortar and pestle
- Filter paper and funnel
- Safety requirements–
- Protective gloves
- Safety goggles
- Lab coat or apron
Procedure of Iodine Test
1. Reagent Preparation
- Potassium iodide (KI) is dissolved in distilled water.
- Elemental iodine crystals is added slowly to the potassium iodide solution and mixed until it dissolves completely.
- The prepared iodine solution is allowed to stand for about 24 hours so that the reagent becomes stable.
- The reagent is stored in a dark coloured bottle and kept in cool and dark place to avoid decomposition of iodine.
2. Procedure of Iodine Test
Procedure for Liquid Samples
- Two clean and dry test tubes are taken and labelled properly.
- About 1 ml of the liquid sample is taken in one test tube.
- About 1 ml of distilled water is taken in another test tube as control.
- Few drops (2–3 drops) of iodine solution is added to both test tubes.
- The contents of the test tubes is mixed gently by shaking.
- The colour change is observed immediately for presence or absence of starch.
Procedure for Solid Samples
- A small piece of the solid sample is taken for testing.
- In case of food materials or fruits a fresh surface is exposed by cutting.
- The sample is placed on a white tile or clean surface.
- Few drops of iodine solution is added directly on the sample.
- The sample is allowed to stand for about one minute.
- The colour change on the surface of the sample is observed carefully.
Procedure for Plant Leaves
- A fresh green leaf is selected and washed with water.
- The leaf is boiled in water for few minutes to kill the cells.
- The boiled leaf is transferred into alcohol and heated using water bath.
- The leaf is kept in alcohol until chlorophyll is removed completely.
- The decolourised leaf is washed with warm water to soften it.
- The leaf is placed on a white tile.
- Few drops of iodine solution is poured over the leaf.
- The colour change in the leaf is observed carefully.
3. Confirmatory Test
- The test tube showing blue–black colour is selected.
- The test tube is placed in a hot water bath.
- The solution is heated gently for few minutes.
- The disappearance of blue–black colour is observed on heating.
- The test tube is removed from water bath and allowed to cool.
- On cooling the reappearance of blue–black colour is observed.
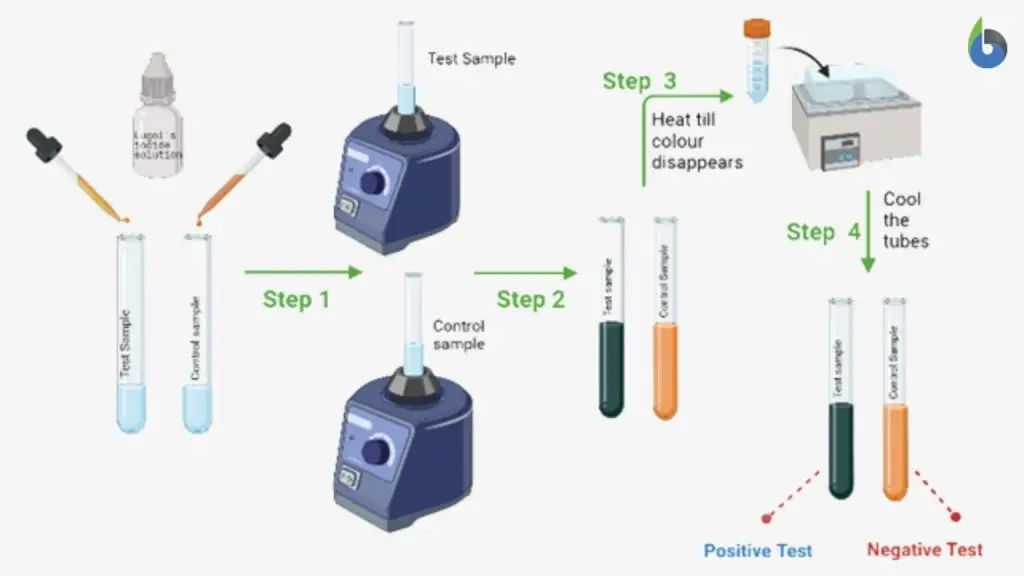
Result and Interpretation of Iodine Test
Positive Result (Presence of Starch)
- The sample shows development of blue–black or dark blue colour.
- This colour formation indicates presence of starch in the given sample.
- It is due to formation of starch–iodine complex where iodine gets trapped inside amylose helix.
- The intensity of colour depends on amount of starch present in the sample.
Negative Result (Absence of Starch)
- No blue–black colour is observed in the sample.
- The solution retains yellow or brown colour of iodine reagent.
- This indicates absence of starch in the given sample.
- Simple sugars like glucose and sucrose do not react with iodine.
Result with Other Polysaccharides
- Amylopectin produces reddish or violet colour due to branched structure.
- Glycogen gives reddish–brown colour when treated with iodine.
- Dextrins may show brown or faint black colour depending on chain length.
- Cellulose does not give any colour change and remains unchanged.
Effect of Different Factors on Iodine test
Effect of Hydrolysis
- On hydrolysis of starch the blue–black colour gradually disappears.
- The colour may change to red and finally yellow.
- This shows breakdown of starch into smaller carbohydrate units.
Effect of Temperature
- On heating the blue–black colour disappears from the solution.
- On cooling the colour reappears again.
- This indicates that iodine test is a reversible reaction.
Agricultural Interpretation
- In unripe fruits the cut surface turns blue–black showing high starch content.
- In ripe fruits little or no colour change is observed due to conversion of starch into sugars.

Uses of Iodine Test
- It is used to detect presence of starch in given sample.
- It is used to differentiate starch from simple carbohydrates like glucose and sucrose.
- It is used to identify different polysaccharides such as glycogen and dextrin.
- It is used to study hydrolysis of starch during enzymatic or acidic digestion.
- It is used in laboratory experiments to demonstrate photosynthesis in plant leaves.
- It is used to check activity of enzyme amylase on starch.
- It is used in microbiology to identify starch hydrolysing bacteria.
- It is used in agriculture to determine ripeness of fruits like apple.
- It is used in food industry for quality control of starch containing products.
- It is used in analytical chemistry as an indicator in iodine titration.
Limitations of Iodine Test
- It is only a qualitative test and does not give exact amount of starch present.
- It gives negative result when starch is hydrolysed into small sugar units.
- It does not react with monosaccharides and disaccharides.
- Other polysaccharides may give different colour and cause confusion.
- The reaction is affected by high temperature and colour may disappear on heating.
- The test does not work properly in acidic conditions.
- The test is also not reliable in alkaline medium.
- Dark coloured samples may interfere with observation of colour change.
- Organic solvents like alcohol may reduce intensity of colour.
- Iodine reagent is toxic and needs careful handling.
Advantages of Iodine Test
- It is a simple and easy test to perform in laboratory.
- It gives quick result with immediate colour change.
- It is highly specific for detection of starch.
- It helps to differentiate starch from simple sugars.
- It can distinguish between different polysaccharides based on colour.
- It is very sensitive and can detect small amount of starch.
- It requires less chemicals and simple apparatus.
- It is cost effective and suitable for routine experiments.
- It is reversible and helps in confirmatory testing.
- It is widely used in biology agriculture and food industry.
FAQ
Q1. What does the iodine test for?
A. The iodine test is used to test presence of starch in a given sample. It mainly detects complex carbohydrate in food materials and plant parts.
Q2. What is the iodine test?
A. Iodine test is a simple chemical test used to identify starch. It is based on colour change produced when iodine solution reacts with starch.
Q3. What is the principle of the iodine test?
A. The principle of iodine test is based on interaction between iodine and amylose present in starch. Iodine fits inside the helical structure of amylose forming a coloured complex.
Q4. What color does iodine turn in the presence of starch?
A. In presence of starch iodine turns blue–black in colour. This colour confirms positive result for starch.
Q5. How do you perform an iodine test for starch?
A. A few drops of iodine solution is added to the sample. Development of blue–black colour indicates presence of starch while no colour change shows absence of starch.
Q6. What are the results and interpretation of the iodine test?
A. Formation of blue–black colour indicates presence of starch. No colour change or retention of yellow colour indicates absence of starch in the sample.
Q7. What are the uses of the iodine test?
A. It is used to detect starch in food samples. It is used in photosynthesis experiments. It is used in agriculture to test fruit ripeness and in laboratories for carbohydrate identification.
Q8. What are the symptoms of iodine deficiency?
A. Iodine deficiency causes goitre enlargement of thyroid gland. Other symptoms include fatigue weight gain dry skin hair loss and reduced mental development in children.
Q9. How can you test your iodine levels (in the human body)?
A. Iodine levels are tested by urine iodine test or blood test. Urine iodine test is commonly used to assess iodine status.
Q10. Who would benefit from testing their iodine levels?
A. Pregnant women children people with thyroid disorders and individuals living in iodine deficient regions benefit from iodine level testing.
Q11. Is the iodine patch test accurate or recommended?
A. The iodine patch test is not accurate and not scientifically recommended. It does not give reliable information about iodine levels in the body.
Q12. What are the different types of iodine tests for humans?
A. Urine iodine concentration test blood iodine test and thyroid function tests are used to assess iodine status in humans.
Q13. Can excess iodine be harmful?
A. Excess iodine intake can cause thyroid dysfunction. It may lead to hyperthyroidism hypothyroidism and thyroid inflammation.
Q14. Why is iodine only used to test for starch and not glucose?
A. Iodine reacts only with helical structure of starch. Glucose lacks this structure so it does not form coloured complex with iodine.
Q15. What are the limitations of the iodine test?
A. It is a qualitative test only. It does not detect simple sugars. Colour change is affected by temperature pH and sample colour.
- Biology Online. (2022, June 16). Iodine test. Biology Online Dictionary. https://www.biologyonline.com/dictionary/iodine-test
- ChemSupply Australia. (2025, August 12). Safety data sheet: Lugol’s iodine solution. https://www.chemsupply.com.au/uploads/sds/JGGNUHMK.pdf
- Croptracker. (n.d.). Starch iodine testing. https://www.croptracker.com/resources/quality-control-resources/starch-iodine-testing.html
- Hanrahan, I., & Galeni, M. (2019, May). Developing a cv. WA 38 starch scale for the Washington State apple industry. WSU Tree Fruit. https://treefruit.wsu.edu/developing-a-cv-wa-38-starch-scale-for-the-washington-state-apple-industry/
- Home Science Tools. (n.d.). Starch test for plants. https://learning-center.homesciencetools.com/article/test-for-starch-photosynthesis/
- HunterLab. (2024, January 4). How to separate and measure the color of starch. https://www.hunterlab.com/blog/top-5-things-to-know-about-measuring-the-color-of-starch/
- Labster. (2025, May 21). 5 creative ways to teach iodine test without lecturing. https://www.labster.com/blog/5-creative-ways-teach-iodine-test
- McIvor, L. M. (2024, September 24). Investigating the need for chlorophyll, light & carbon dioxide. Save My Exams. https://www.savemyexams.com/igcse/biology/cie/23/revision-notes/6-plant-nutrition/6-1-photosynthesis-and-leaf-structure/6-1-5-investigating-the-need-for-chlorophyll-light-and-carbon-dioxide/
- McIvor, L. M. (2025, November 16). Practical: Food tests. Save My Exams. https://www.savemyexams.com/igcse/biology/edexcel/19/revision-notes/2-structure-and-function-in-living-organisms/biological-molecules/practical-food-tests/
- Ophardt, C. (2022, July 4). Starch and iodine. Chemistry LibreTexts. https://chem.libretexts.org/Bookshelves/Biological_Chemistry/Supplemental_Modules_(Biological_Chemistry)/Carbohydrates/Case_Studies/Starch_and_Iodine
- Practical Biology. (n.d.). Investigating the effect of pH on amylase activity. https://practicalbiology.org/bio-molecules/factors-affecting-enzyme-activity/investigating-the-effect-of-ph-on-amylase-activity
- Ricca Chemical Company. (n.d.). Starch. https://www.riccachemical.com/pages/tech-tips/starch
Ruppersberg, K., Rautenstrauch, H., & Thomsen, S. (2022). Know thy carbs! Safer carbohydrate detection methods for school labs: Part 2. ChemistryViews. https://doi.org/10.25656/01:29733 - Sapkota, A. (2024, October 28). Iodine test: Principle, procedure, result, uses. Microbe Notes. https://microbenotes.com/iodine-test/
- Sulistyarti, H., Atikah, A., Fardiyah, Q., & Asdauna, A. (2015, July). The effect of pH on the absorbance of starch-iodine [Figure]. ResearchGate. https://www.researchgate.net/figure/The-Effect-of-pH-on-the-Absorbance-of-Starch-iodine_fig2_294721237
- The iodine-starch complex: A comprehensive review of principles, analytical procedures, and modern applications in chemistry and biology. (n.d.). [Review article].
- wikiHow Staff. (2025, January 26). 3 ways to prepare iodine solution. wikiHow. https://www.wikihow.com/Prepare-Iodine-Solution
- Wikipedia contributors. (n.d.). Iodine–starch test. Wikipedia. Retrieved from https://en.wikipedia.org/wiki/Iodine%E2%80%93starch_test