What is IMViC test?
The IMViC test is a group of biochemical tests which is used for the identification and differentiation of bacteria belonging to the family Enterobacteriaceae. It is mainly used to distinguish fecal coliform bacteria from non-fecal coliform bacteria. The term IMViC is an acronym which stands for Indole, Methyl Red, Voges–Proskauer and Citrate test, where the small letter “i” is added only for easy pronunciation. This test is commonly used in microbiology laboratories for diagnostic and water quality analysis.
The principle of IMViC test is based on different metabolic activities of bacteria. Different organisms utilize nutrients through different biochemical pathways and the end products formed varies among them. These variations are detected by specific reagents used in each test. The combined results of all four tests give a characteristic pattern which is helpful in bacterial identification.
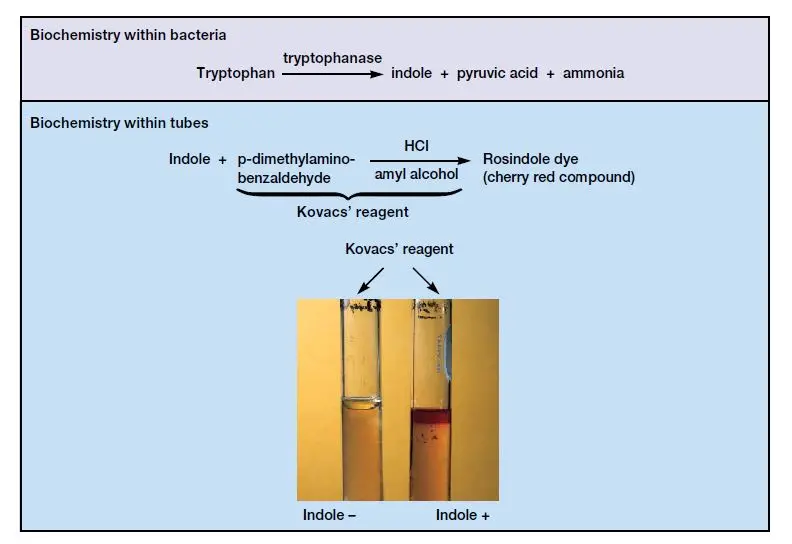
The Indole test is used to detect the ability of an organism to degrade the amino acid tryptophan. This process occurs when the bacteria produces the enzyme tryptophanase which breaks down tryptophan into indole, pyruvic acid and ammonia. The indole formed reacts with the reagent to produce a colored ring indicating a positive result.
The Methyl Red test is used to identify bacteria that produce stable acidic end products during glucose fermentation. In this test mixed acids are produced and the pH of medium is lowered. The methyl red indicator remains red under acidic conditions which shows a positive test. This indicates that the organism follows the mixed acid fermentation pathway.
The Voges–Proskauer test is used to detect organisms that produce neutral end products like acetoin during glucose fermentation. This test identifies bacteria that do not produce stable acids but instead convert glucose into neutral compounds. The reaction is as follows– acetoin is oxidized in the presence of reagents to form a red colored compound which indicates a positive result.
The Citrate test determines the ability of an organism to use citrate as the sole source of carbon. In this test citrate is utilized and converted into alkaline products. Due to this change the color of the medium changes indicating a positive test. This shows that the organism possess citrate permease enzyme.
The IMViC tests are interpreted together because some of the results are related to each other. The Methyl Red and Voges–Proskauer tests are usually opposite in nature. For example Escherichia coli gives positive result for Indole and Methyl Red test but negative for Voges–Proskauer and Citrate test (++––). On the other hand Enterobacter and Klebsiella species generally show negative results for Indole and Methyl Red but positive for Voges–Proskauer and Citrate test (––++). These patterns are used as a standard method for bacterial identification.
Principle of IMViC Test
The principle of IMViC test is based on the difference in metabolic activities of bacteria belonging to the family Enterobacteriaceae. These bacteria differ in the way they ferment carbohydrates and utilize specific substrates. The IMViC test includes four biochemical reactions namely Indole, Methyl Red, Voges–Proskauer and Citrate test, which together provide a metabolic pattern useful for identification. The small letter “i” is included only for easy pronunciation of the term.
The principle of Indole test is based on the ability of certain bacteria to produce the enzyme tryptophanase. This enzyme helps in the breakdown of the amino acid tryptophan into indole, pyruvic acid and ammonia. The indole produced accumulates in the medium and reacts with Kovac’s reagent to form a colored compound. The formation of a red or pink colored ring indicates the presence of indole.
The principle of Methyl Red test depends on the production of stable acidic end products during glucose fermentation. Some bacteria ferment glucose by mixed acid pathway producing large amount of acids. These acids lower the pH of the medium beyond the buffering capacity. When methyl red indicator is added, it remains red in acidic condition showing a positive result.
The principle of Voges–Proskauer test is based on the detection of neutral end products of glucose fermentation. Some organisms convert glucose into neutral compounds like acetoin instead of acids. Acetoin is oxidized in the presence of specific reagents to form a colored complex. The development of pink or red color indicates a positive Voges–Proskauer reaction.
The principle of Citrate test is based on the ability of bacteria to utilize citrate as the sole source of carbon. Organisms having citrate permease can transport and metabolize citrate present in the medium. During this process alkaline products are formed which increases the pH of the medium. Due to this change the indicator changes its color, indicating a positive citrate utilization test.
Objectives of IMViC Test
- To differentiate bacteria belonging to the family Enterobacteriaceae based on their biochemical characters.
- To distinguish fecal coliform bacteria such as Escherichia coli from non-fecal or soil coliform bacteria like Enterobacter and Klebsiella species.
- To identify unknown bacteria by studying their metabolic properties such as indole production acid production acetoin formation and citrate utilization.
- To study the ability of bacteria to ferment glucose by mixed acid pathway or by butylene glycol pathway.
- To use IMViC test as a primary screening test in microbiology laboratory for presumptive identification of organisms.
- To help in sanitary and public health analysis by detecting indicator organisms in water and food samples.
Requirements for IMViC Test
The requirements for IMViC test are listed below according to each individual test–
- Indole Test
– Tryptophan broth or peptone water
– SIM medium or MIU medium (optional)
– Kovac’s reagent
– Ehrlich’s reagent
– Sterile inoculating loop or inoculating needle - Methyl Red Test
– MR-VP broth (glucose phosphate broth)
– Methyl red indicator
– Sterile inoculating loop or needle - Voges-Proskauer Test
– MR-VP broth
– Alpha-naphthol (VP reagent A)
– Potassium hydroxide (VP reagent B)
– Clean test tubes for shaking - Citrate Utilization Test
– Simmons citrate agar slant
– Bromothymol blue indicator (present in medium)
– Sterile inoculating needle - General Laboratory Requirements
– Pure bacterial cultures
– Incubator maintained at 35–37°C
– Bunsen burner or spirit lamp for aseptic technique
IMViC test Procedure
The IMViC test Procedure is accomplished with these 4 tests; Indole Test, Methyl Red Test, Voges-Proskauer Test and Citrate Utilization Test
1. Indole test
What is Indole Test?
The Indole test is a biochemical test that is used to detect the ability of bacteria to produce indole from the amino acid tryptophan. It is based on the presence of the enzyme tryptophanase which hydrolyzes tryptophan into indole pyruvic acid and ammonia. When the organism is grown in a tryptophan rich medium such as peptone water or tryptone broth the indole is released and remains in the medium as a waste product. After incubation Kovac’s reagent (or Ehrlich’s reagent) is added and if indole is present a cherry red or pink coloured ring is formed at the surface of the medium while absence of colour indicates a negative result. This test is an important part of IMViC tests and is used to differentiate indole positive bacteria like Escherichia coli from indole negative organisms such as Klebsiella and Enterobacter.
Principle of Indole Test

The principle of Indole test is based on the ability of certain bacteria to produce the enzyme tryptophanase. It is the enzyme which helps in the breakdown of the amino acid tryptophan present in the medium such as peptone water or tryptone broth. During this process tryptophan is hydrolyzed into indole pyruvic acid and ammonia, in which pyruvic acid and ammonia is utilized by the bacterial cell but indole is left unused in the medium. After incubation Kovac’s reagent is added which reacts with the indole to form a coloured quinoidal compound, and this results in the formation of a cherry red or pink coloured ring at the top of the medium indicating a positive reaction.
Procedure of Indole Test
The procedure of Indole test is carried out by following the steps given below–
- Inoculation– The test organism is inoculated into a tryptophan rich medium such as peptone water or tryptone broth using a sterile inoculating loop or needle. In case of SIM medium the inoculating needle is stabbed into the agar deep up to about three fourth of the depth.
- Incubation– The inoculated tubes are incubated at 35–37°C for a period of 24 to 48 hours. During this time the bacteria grows and utilizes the tryptophan present in the medium.
- Addition of reagent– After incubation few drops of Kovac’s reagent is added carefully along the side of the test tube. Ehrlich’s reagent may be used instead when anaerobic organisms are tested.
- Observation of reaction– The tube is allowed to stand for about 1–2 minutes without shaking so that the reagent forms a separate layer at the top of the medium.
- Interpretation of result– Formation of a cherry red or pink coloured ring at the top indicates a positive Indole test. Absence of colour change or formation of yellow coloured layer indicates a negative Indole test.
Indole Test Positive (+) and Negative (–) Bacteria
Positive (+)
- Escherichia coli
- Klebsiella oxytoca
- Proteus vulgaris
- Morganella morganii
- Citrobacter koseri
- Vibrio cholerae
- Providencia species
Negative (–)
- Klebsiella pneumoniae
- Enterobacter aerogenes
- Proteus mirabilis
- Citrobacter freundii
- Salmonella species
- Shigella species
- Pseudomonas aeruginosa
- Serratia marcescens
2. Methyl red test
What is Methyl red test?
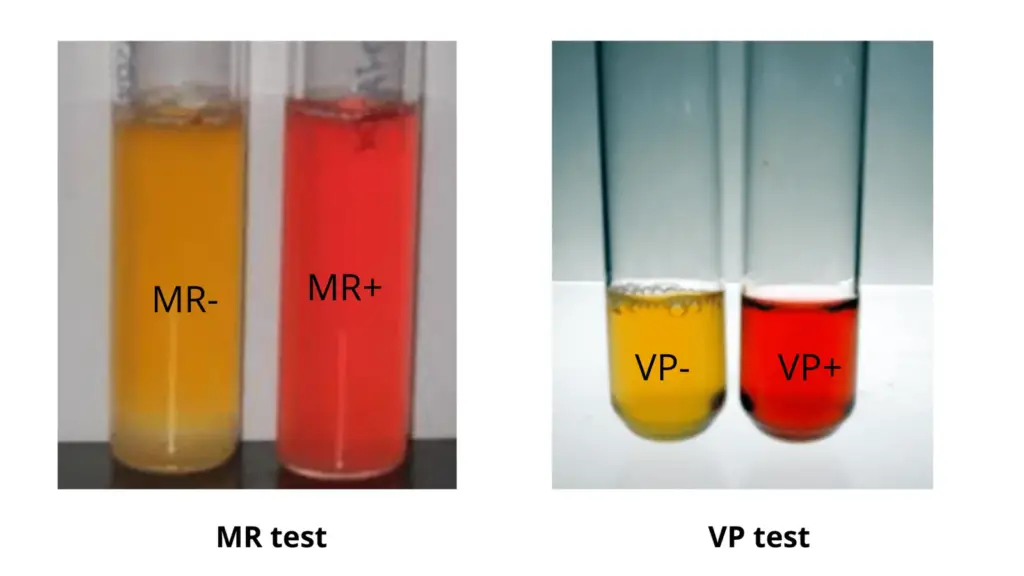
The Methyl red test is a biochemical test that is used to determine the ability of bacteria to carry out mixed acid fermentation of glucose. It is performed using MR-VP broth which contains glucose and a phosphate buffer. When the organism ferments glucose by mixed acid pathway large amount of stable acids such as lactic acid acetic acid and formic acid is produced which lowers the pH of the medium below 4.4 and overcomes the buffering action. After incubation for about 48 hours methyl red indicator is added to the culture and if strong acid is present the medium turns red indicating a positive result, while yellow colour indicates a negative test due to production of neutral end products through butylene glycol pathway. This test is commonly used to differentiate MR positive organisms like Escherichia coli from MR negative bacteria such as Klebsiella and Enterobacter.
Principle of Methyl red test
The principle of Methyl red test is based on the ability of certain bacteria to ferment glucose by mixed acid fermentation pathway. When these organisms are grown in glucose phosphate broth they produce large amount of stable acids such as lactic acid acetic acid and formic acid. These acids are sufficient to overcome the buffering action of phosphate present in the medium and cause a sharp fall in pH up to 4.4 or below. After incubation for about 48 hours methyl red indicator is added to the culture and if the medium remains highly acidic it turns red indicating a positive test, while yellow colour indicates a negative result due to production of neutral end products and maintenance of higher pH.
Procedure of Methyl Red Test
The procedure of Methyl red test is performed by following the steps given below–
- Inoculation– A tube containing MR-VP broth (glucose phosphate broth) is inoculated with the test organism using a sterile inoculating loop or needle. The medium contains glucose peptone and phosphate buffer which are required for fermentation.
- Incubation– The inoculated broth is incubated at 35–37°C for a minimum period of 48 hours. This incubation period is important to allow complete fermentation and stabilization of acidic or neutral end products.
- Addition of reagent– After incubation about 5–6 drops of methyl red indicator is added directly into the broth culture.
- Observation of result– The colour change is observed immediately after adding the indicator. Development of a bright red colour indicates a positive Methyl red test, while yellow or orange colour indicates a negative test due to production of neutral end products.
Methyl Red (MR) Test Positive (+) and Negative (–) Bacteria
Positive (+)
- Escherichia coli
- Proteus vulgaris
- Proteus mirabilis
- Salmonella species
- Shigella species
- Citrobacter freundii
- Yersinia species
- Staphylococcus aureus
Negative (–)
- Klebsiella pneumoniae
- Klebsiella oxytoca
- Enterobacter aerogenes
- Serratia marcescens
- Hafnia species
3. Voges-Proskauer (VP ) Test

What is Voges-Proskauer (VP ) Test?
The Voges-Proskauer test is a biochemical test used to determine the ability of bacteria to ferment glucose by butylene glycol pathway. It is a part of IMViC tests and is mainly used to differentiate organisms based on the end products formed during glucose fermentation. In this test the organism is grown in MR-VP broth where glucose is metabolized and instead of producing strong stable acids the bacteria converts pyruvate into a neutral compound called acetoin (acetylmethylcarbinol). After incubation Barritt’s reagents that is alpha-naphthol and potassium hydroxide are added, and in presence of oxygen acetoin is oxidized to diacetyl which reacts with guanidine compounds of the medium to produce a pink to red colour at the top of the broth indicating a positive result. Absence of red colour indicates a negative VP test. This test helps in differentiating VP positive organisms such as Klebsiella and Enterobacter from VP negative bacteria like Escherichia coli.
Principle of Voges Proskauer test
The principle of Voges-Proskauer test is based on the ability of certain bacteria to ferment glucose by butylene glycol pathway. In this pathway glucose is metabolized to pyruvate and further converted into neutral end products such as acetoin instead of producing strong acids. The acetoin formed during fermentation is detected by adding Barritt’s reagents namely alpha-naphthol and potassium hydroxide to the MR-VP broth after incubation. In presence of oxygen the acetoin is oxidized to diacetyl, which then reacts with guanidine compounds present in the peptone of the medium to form a pinkish red coloured complex. Development of red colour indicates a positive VP test while absence of colour or copper colour indicates a negative result.
Procedure of Voges Proskauer test
The procedure of Voges Proskauer test is carried out by following the steps given below–
- Inoculation– A tube containing MR-VP broth is inoculated with the test organism using a sterile inoculating loop or needle. The medium contains glucose peptone and phosphate buffer which are required for fermentation.
- Incubation– The inoculated broth is incubated at 35–37°C for about 48 hours. This incubation period is necessary for the accumulation of acetoin if the organism follows butylene glycol pathway.
- Addition of reagents– After incubation about 1–2 ml of the culture is transferred into a clean test tube. First alpha-naphthol (VP reagent A) is added followed by potassium hydroxide (VP reagent B) in required amount.
- Aeration– The tube is shaken vigorously to introduce oxygen into the medium which helps in oxidation of acetoin to diacetyl.
- Observation of result– The tube is allowed to stand for 15–30 minutes for colour development. Formation of pink to red colour at the surface indicates a positive Voges Proskauer test, while absence of red colour or development of copper brown colour indicates a negative result.
Voges–Proskauer (VP) Test Positive (+) and Negative (–) Bacteria
Positive (+)
- Klebsiella pneumoniae
- Klebsiella oxytoca
- Enterobacter aerogenes
- Serratia marcescens
- Proteus mirabilis
- Hafnia species
- Staphylococcus aureus
Negative (–)
- Escherichia coli
- Proteus vulgaris
- Citrobacter freundii
- Salmonella species
- Shigella species
4. Citrate utilization test

What is Citrate utilization test?
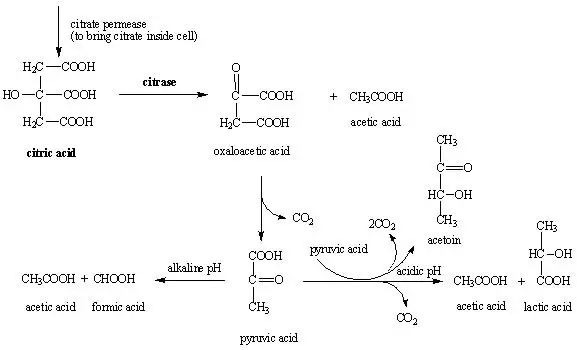
The Citrate utilization test is a biochemical test used to determine the ability of bacteria to utilize citrate as the sole source of carbon and ammonium salts as the sole source of nitrogen. It is performed using Simmons citrate agar which is a defined medium containing sodium citrate ammonium salts and the pH indicator bromothymol blue. Organisms that possess the enzyme citrate permease are able to transport citrate into the cell and utilize it during metabolism, resulting in the production of alkaline substances such as ammonia and sodium carbonate. This increase in alkalinity raises the pH of the medium and causes the colour to change from green to blue indicating a positive citrate test. Absence of growth with no colour change indicates a negative result. This test is commonly used in IMViC tests to differentiate citrate positive organisms like Klebsiella and Enterobacter from citrate negative bacteria such as Escherichia coli.
Principle of Citrate utilization test
The principle of Citrate utilization test is based on the ability of certain bacteria to use citrate as the sole source of carbon and ammonium salts as the sole source of nitrogen. When the organism possesses the enzyme citrate permease the citrate is transported into the cell and broken down by citrase into simpler compounds which finally produces carbon dioxide. The carbon dioxide reacts with sodium present in the medium to form sodium carbonate and at the same time ammonium salts are metabolized to release ammonia, both of which makes the medium alkaline. This rise in pH causes the indicator bromothymol blue present in Simmons citrate agar to change its colour from green to blue. Development of blue colour with visible growth indicates a positive citrate test, while absence of growth and no colour change indicates a negative result.
Procedure of citrate utilization test
The procedure of Citrate utilization test is carried out by following the steps given below–
- Preparation and inoculation – A sterile tube containing Simmons citrate agar slant is taken. Using a sterile inoculating needle a small amount of the test organism from a pure culture is picked. The organism is lightly streaked over the surface of the agar slant, taking care to avoid heavy inoculation.
- Incubation– The inoculated tube is incubated at 35–37°C for 24 to 48 hours. The cap of the tube is kept loose to allow proper aeration as citrate utilization is an aerobic process.
- Observation of growth– After incubation the slant is observed for visible growth of the organism on the surface of the medium.
- Interpretation of result– A change in colour of the medium from green to blue with growth indicates a positive citrate utilization test. Absence of growth with no colour change and the medium remaining green indicates a negative citrate utilization test.
Citrate Utilization Test Positive (+) and Negative (–) Bacteria
Positive (+)
- Klebsiella pneumoniae
- Klebsiella oxytoca
- Enterobacter aerogenes
- Citrobacter freundii
- Salmonella Typhimurium
- Serratia marcescens
- Proteus mirabilis
- Pseudomonas aeruginosa
- Providencia species
Negative (–)
- Escherichia coli
- Shigella species
- Salmonella Typhi
- Salmonella Paratyphi A
- Morganella morganii
- Yersinia enterocolitica
IMVIC test results
| S. No | Test Name | Positive result | Negative Result |
|---|---|---|---|
| 1. | Indole Test | color changes pink to red (“cherry-red ring”) | no color change occurs |
| 2. | Methyl Red test | a stable red color develops | a yellow color develops |
| 3. | Voges Proskauer Test | a pink-red color develops | a yellow color develops |
| 4. | Citrate Test | develops blue color from green | No color change |
Indole Test
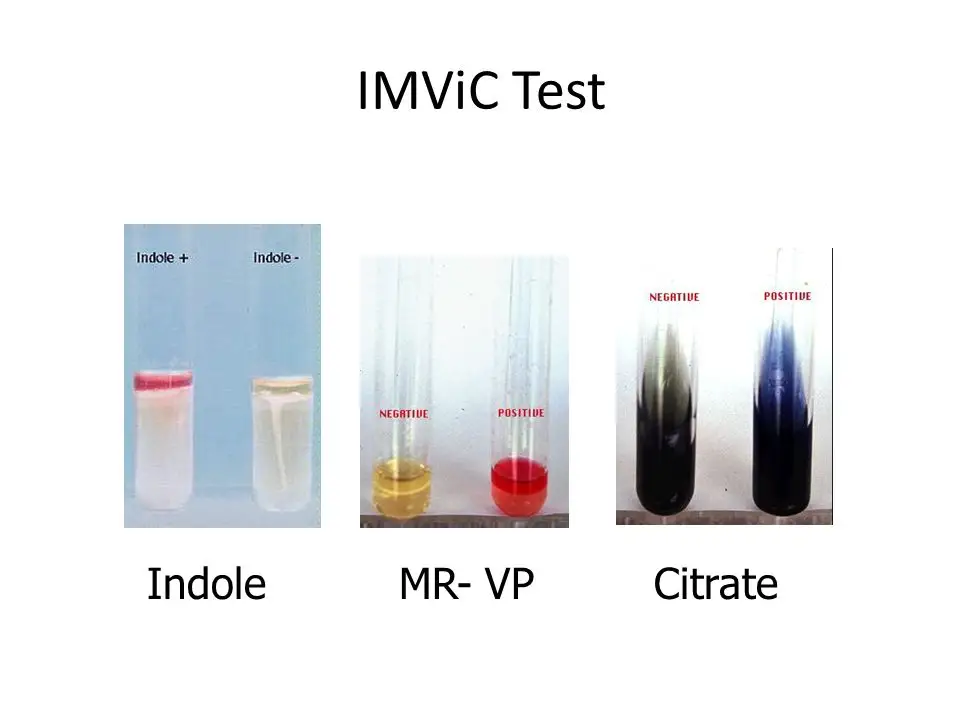
It is the test used to determine the ability of an organism to produce indole from tryptophan. In a positive indole test result, a distinct red or pink coloured ring is formed at the top liquid layer of the broth after the addition of Kovac’s reagent. This indicates the presence of enzyme tryptophanase which hydrolyses tryptophan into indole. Escherichia coli and Proteus vulgaris shows positive indole test reaction.
In a negative indole test result, the reagent layer remains yellow or brownish in colour and no ring is observed at the surface. This indicates that indole is not produced by the organism. Klebsiella pneumoniae and Enterobacter aerogenes shows negative indole test reaction.
Methyl Red (MR) Test
It is the test used to detect the production of stable acidic end products from glucose fermentation. In a positive methyl red test, the medium turns bright red immediately after the addition of methyl red indicator. This indicates that the organism performs mixed acid fermentation and lowers the pH to 4.4 or below. Escherichia coli, Proteus vulgaris and Salmonella species shows positive MR test result.
In a negative MR test result, the medium remains yellow or sometimes orange in colour. This indicates that the pH is above 6.0 and stable acids are not produced. Instead, neutral end products are formed. Klebsiella pneumoniae and Enterobacter aerogenes shows negative methyl red test.
Voges–Proskauer (VP) Test
It is the test used to detect the production of acetoin during glucose fermentation. In a positive VP test result, a pink to red colour develops at the surface of the medium within 15–30 minutes after the addition of Barritt’s reagents (α-naphthol and KOH) and shaking. This indicates the presence of acetoin which is a precursor of 2,3-butanediol fermentation. Klebsiella pneumoniae, Enterobacter aerogenes and Serratia marcescens gives positive VP test.
In a negative VP test result, no red colour is produced and the medium may remain unchanged or turns copper or brownish in colour. This indicates the absence of acetoin production. Escherichia coli and Proteus vulgaris shows negative VP test reaction.
Citrate Utilization Test
It is the test used to determine the ability of organism to utilize citrate as the sole carbon source. In a positive citrate test, visible growth is observed on the agar slant and the colour of the medium changes from green to deep Prussian blue. This indicates that citrate is utilized and alkaline by-products are produced which raises the pH. Klebsiella pneumoniae, Enterobacter aerogenes and Citrobacter freundii shows positive citrate utilization test.
In a negative citrate test result, no growth is observed and the medium remains green in colour. This indicates that citrate is not utilized by the organism. Escherichia coli and Shigella species gives negative citrate test result.
E coli imvic test results
Escherichia coli (E. coli) is a gram-negative, rod-shaped bacterium that is commonly found in the human gastrointestinal tract. The results of the IMViC test (Indole production, Methyl red, Voges-Proskauer, Citrate utilization) for E. coli are typically as follows:
- Indole production test: Positive result
- Methyl red test: Positive result
- Voges-Proskauer (VP) test: Negative result
- Citrate utilization test: Negative result
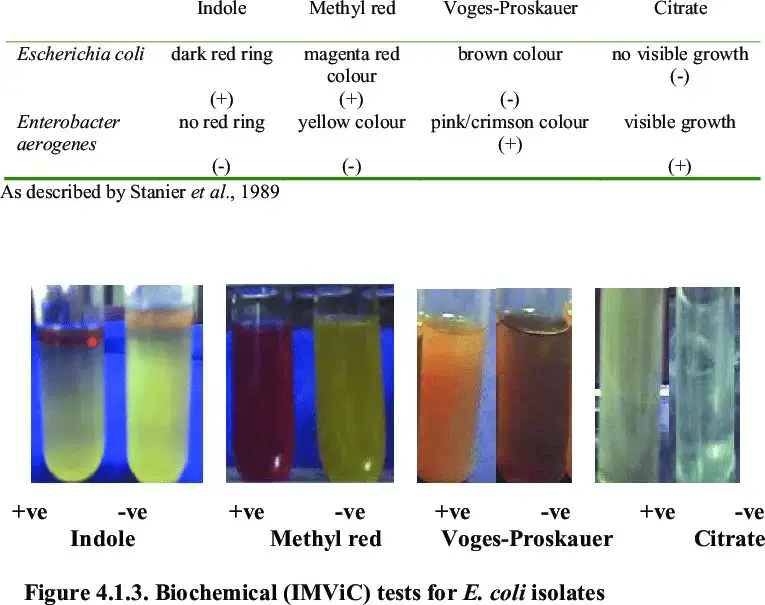
IMViC Test – Positive and Negative Result Bacteria
| Test | Positive (+) Bacteria | Negative (–) Bacteria |
|---|---|---|
| Indole Test | Escherichia coli Proteus vulgaris Klebsiella oxytoca Morganella morganii Citrobacter koseri | Klebsiella pneumoniae Enterobacter aerogenes Proteus mirabilis Citrobacter freundii Salmonella species |
| Methyl Red (MR) Test | Escherichia coli Proteus vulgaris Proteus mirabilis Salmonella species Shigella species Citrobacter freundii | Klebsiella pneumoniae Klebsiella oxytoca Enterobacter aerogenes Serratia marcescens |
| Voges Proskauer (VP) Test | Klebsiella pneumoniae Klebsiella oxytoca Enterobacter aerogenes Serratia marcescens Proteus mirabilis | Escherichia coli Proteus vulgaris Citrobacter freundii Salmonella species Shigella species |
| Citrate Utilization Test | Klebsiella pneumoniae Klebsiella oxytoca Enterobacter aerogenes Citrobacter freundii Salmonella Typhimurium Proteus mirabilis | Escherichia coli Shigella species Salmonella Typhi Morganella morganii |
Common IMViC Test Profiles
- ++–
Indole (+), MR (+), VP (–), Citrate (–)
Typical organisms are Escherichia coli and Proteus vulgaris. - –++
Indole (–), MR (–), VP (+), Citrate (+)
Typical organisms are Klebsiella pneumoniae, Enterobacter aerogenes and Serratia marcescens. - -+-+
Indole (–), MR (+), VP (–), Citrate (+)
Typical organisms are Salmonella species, Citrobacter freundii and Proteus mirabilis.
Uses of IMViC Test
- It is used for differentiation and identification of members of Enterobacteriaceae family such as Escherichia, Klebsiella, Enterobacter, Salmonella and Proteus.
- It is used in sanitary analysis of water and food to distinguish fecal coliforms (Escherichia coli) from non-fecal or soil coliforms like Enterobacter and Klebsiella.
- It is used in clinical laboratories for presumptive identification of enteric pathogens responsible for infections like urinary tract infection and gastroenteritis.
- It is used for species level differentiation within closely related genera.
For example– Indole test differentiates Klebsiella oxytoca (positive) from Klebsiella pneumoniae (negative).
Indole and citrate test helps in differentiating Citrobacter koseri from Citrobacter freundii.
Indole test helps in differentiating Proteus vulgaris from Proteus mirabilis. - It is used in teaching laboratories to demonstrate metabolic characteristics and biochemical diversity of bacteria.
- It is used in research studies related to bacterial physiology and metabolic pathways.
- It is used as a quality control test where methyl red test helps in checking contamination and pH stability of culture media.
- It is used in food and fermentation industries where Voges–Proskauer test helps in detection of spoilage organisms and acetoin producing bacteria.
Limitations of IMVIC Test
- It is mainly a presumptive test and cannot identify bacteria accurately up to species level without performing additional biochemical or serological tests.
- Different bacterial genera may show similar or overlapping IMViC patterns which makes interpretation confusing.
For example– Escherichia coli and Proteus vulgaris shows same IMViC pattern (++–) and needs urease test for confirmation.
Citrobacter freundii and many Salmonella species also shows similar pattern (-+-+) requiring further tests. - It is a time consuming procedure as these tests are culture based and require longer incubation period for reliable results.
- Methyl red test requires minimum 48 hours of incubation and early reading may give false positive results.
- The test results are highly affected by technical errors during procedure.
Heavy inoculum in citrate test may carry nutrients and gives false positive result.
Improper shaking in VP test may result in false negative reaction.
Old or unstable reagents may produce incorrect colour reactions. - Some bacterial species or strains may show variable reactions deviating from standard IMViC patterns which may complicate identification.
- The test is limited only to culturable organisms and cannot be used for identification of non-culturable bacteria.
Advantages of IMViC Test
- It is useful for effective differentiation of Enterobacteriaceae family and helps in separating fecal coliforms like Escherichia coli from non-fecal coliforms such as Klebsiella and Enterobacter.
- It is a cost effective biochemical test when compared to advanced molecular identification techniques.
- It provides results within short period of time usually within 24 to 48 hours which helps in early presumptive identification.
- It is simple to perform and interpret as it requires basic culture media and simple reagents.
- It is very important in sanitary analysis of water and food as it helps in detecting fecal contamination.
- It gives clear metabolic differentiation as methyl red and Voges–Proskauer test detects different fermentation pathways.
- It can be performed along with combined media like SIM medium where indole production can be studied along with motility and hydrogen sulphide production.
FAQ
1. What is the IMViC test?
Answer:
The IMViC test is a group of biochemical tests used for identification and differentiation of Gram negative enteric bacteria. It is mainly used to differentiate coliform bacteria based on their metabolic activities.
2. What does IMViC stand for?
Answer:
IMViC stands for Indole test, Methyl red test, Voges Proskauer test, and Citrate utilization test.
3. What are the four tests included in the IMViC series?
Answer:
The four tests included in IMViC series are Indole test, Methyl red test, Voges Proskauer test, and Citrate utilization test.
4. What is the purpose of the IMViC test?
Answer:
The purpose of IMViC test is to differentiate members of Enterobacteriaceae based on their ability to produce different metabolic end products during glucose fermentation.
5. What is the principle behind the IMViC tests?
Answer:
The principle of IMViC tests is based on detection of specific metabolic reactions such as indole production, mixed acid fermentation, acetoin production, and citrate utilization by bacteria.
6. How are IMViC tests performed?
Answer:
IMViC tests are performed by inoculating the test organism into specific culture media followed by incubation and addition of appropriate reagents to observe colour change or growth.
7. How are IMViC test results interpreted?
Answer:
IMViC test results are interpreted based on colour change or growth observed in each test, and the combined pattern of positive and negative reactions is used for identification of bacteria.
8. What reagents are used in the IMViC tests?
Answer:
The reagents used in IMViC tests are Kovac’s reagent for Indole test, methyl red indicator for MR test, alpha-naphthol and potassium hydroxide for VP test, and bromothymol blue present in Simmons citrate agar for citrate test.
9. What is the Indole test and its principle?
Answer:
The Indole test is used to detect the ability of bacteria to produce indole from tryptophan. Its principle is based on the action of enzyme tryptophanase which breaks tryptophan into indole that reacts with Kovac’s reagent to produce red colour.
10. What is the Methyl Red (MR) test and its principle?
Answer:
The Methyl red test detects mixed acid fermentation of glucose. Its principle is based on production of stable acids which lower the pH below 4.4 and turn methyl red indicator red.
11. What is the Voges-Proskauer (VP) test and its principle?
Answer:
The Voges Proskauer test detects acetoin production from glucose fermentation. Its principle is based on oxidation of acetoin to diacetyl which reacts with reagents to produce pink to red colour.
12. What is the Citrate Utilization test and its principle?
Answer:
The Citrate utilization test determines the ability of bacteria to use citrate as sole carbon source. Its principle is based on alkaline product formation which changes bromothymol blue indicator from green to blue.
13. What are the applications of IMViC tests in microbiology?
Answer:
IMViC tests are used in identification of enteric bacteria, differentiation of coliforms, water quality testing, and clinical diagnostic microbiology.
14. What are the typical IMViC results for Escherichia coli?
Answer:
The typical IMViC results for Escherichia coli are Indole positive, Methyl red positive, Voges Proskauer negative, and Citrate negative (++–).
15. What are the typical IMViC results for Klebsiella pneumoniae?
Answer:
The typical IMViC results for Klebsiella pneumoniae are Indole negative, Methyl red negative, Voges Proskauer positive, and Citrate positive (–++).
- American Society for Microbiology. (2009, December 1). Citrate test.
- Aryal, S. (2022, August 10). Simmons citrate agar- Composition, principle, uses, preparation and result interpretation. Microbiology Info.
- BYJU’S. (n.d.). Difference between E.Coli and Klebsiella.
- Cooper, C. R., Jr. (2019). Sulfide-indole-motility (SIM) test. Youngstown State University.
- Dahal, P. (2024, May 4). IMViC test: Principle, result chart, examples, uses. Microbe Notes.
- Guentzel, M. N. (1996). Escherichia, Klebsiella, Enterobacter, Serratia, Citrobacter, and Proteus. In S. Baron (Ed.), Medical microbiology (4th ed.). University of Texas Medical Branch at Galveston.
- Hafezi, A., & Khamar, Z. (2024). The method and analysis of some biochemical tests commonly used for microbial identification: A review. Comprehensive Health and Biomedical Studies, 3(2), e160199. https://doi.org/10.5812/chbs-160199
- Microrao. (2006, June). IMViC reactions.
- Orbit Biotech. (2023, April 23). IMViC test.
- Rama University. (n.d.). Biochemical test: IMViC reactions.
- Sigma-Aldrich. (2018). 85438 SIM medium (Sulfide indole motility medium).
- Smith, M., & Selby, S. (2021, March 19). 3.9: Simmons citrate agar. Biology LibreTexts.The IMViC test series: A comprehensive analysis of biochemical principles, standardized procedures, and diagnostic differentiation of Enterobacteriaceae. (n.d.).
- University of Mustansiriyah. (n.d.). Bacterial diagnosis (Gram negative bacteria) 1- IMViC test.
- VUMIE. (n.d.). Citrate utilization test (Simmons).
سلام،ممنون بابت مطالب فوق العاده عالی،خیلی کمک کننده بود برای من