Astrovirus is a type of virus that can cause gastroenteritis (also known as stomach flu) in humans. The virus is highly contagious and is primarily spread through close contact with infected individuals or through contaminated food or water. Symptoms of astrovirus infection may include diarrhea, vomiting, abdominal pain, and fever. Most people recover from an astrovirus infection within a few days without needing any specific treatment, although severe cases may require hospitalization. If you think you may have an astrovirus infection, it is important to practice good hygiene, such as washing your hands frequently and disinfecting surfaces, to prevent the spread of the virus to others.
Human Astrovirus
- Positive-sense, single-stranded RNA viruses were discovered in 1975 as human astroviruses (HAtVs). Astroviruses infecting other species, namely mammals and birds, have been found and classified into the genera Mamastrovirus and Avastrovirus.
- Through next-generation sequencing, numerous new astroviruses infecting various species, including humans, have been characterised, and the Astroviridae family demonstrates a high degree of diversity and zoonotic potential.
- There are three distinct types of HAstVs: the classic (MAstV 1), HAstV-MLB (MAstV 6), and HAstV-VA/HMO (MAstVs 8 and 9).
- Classic HAstVs have eight serotypes and contribute for 2% to 9% of all acute nonbacterial gastroenteritis in children around the world.
- In general, infections are self-limiting, but in immunocompromised patients they can spread systemically and produce serious illnesses.
- Other categories have also been discovered in children with gastroenteritis; however, extraintestinal diseases have also been proposed for them.
- Classic HAstVs can be grown in cells, allowing for the investigation of their cell cycle, which is comparable to that of caliciviruses.
- The continual appearance of new astroviruses with a potential for zoonotic transmission underscores the need to gain insights into their biology to prevent future health hazards.
Taxonomy and Classification
- The International Committee on Taxonomy of Viruses has recently categorised astroviruses as an unique family of plus-sense, single-stranded (ss) RNA viruses, the Astroviridae.
- The family comprises of a single genus, Astrovirus, and the type strain is human astrovirus type 1. The host species of origin is used to identify virus species (e.g., human astrovirus [HAstV], bovine astrovirus [BAstV]), whereas serotypes are recognised by number (e.g. HAstV-1).
Host Range and Virus Propagation
- Astroviruses have been identified in numerous mammalian species and a few bird species, with each host species enabling the reproduction of a distinct virus species.
- Human astroviruses can replicate in a monkey cell line, although direct infection of primates with human astrovirus strains has not been documented.
- When investigated, the serum of animals infected with a homologous virus does not respond with heterologous viruses from different species.
- The bovine and ovine astroviruses have been propagated in experimentally infected gnotobiotic animals, with only the latter species exhibiting symptomatic illness.
- Human, bovine, and porcine astroviruses have been adapted for growth in cell culture, with the use of host-derived cell lines and the addition of trypsin to the growth medium.
- EpidemiologyCaco-2 is commonly utilised for the main isolation and multiplication of human astrovirus strains.
Geographic and Seasonal Distribution
In temperate areas, human astroviruses have a characteristic winter seasonality and are dispersed globally. Less is known about animal astroviruses, however strains have been identified in all regions where intensive searches have been conducted.
Structure of Human Astrovirus
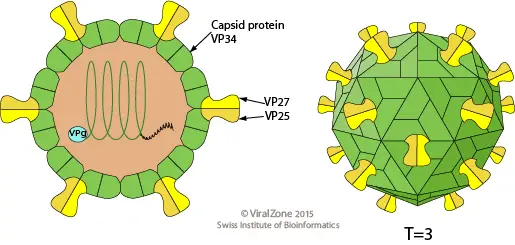
- Five or six spots make up the star-like appearance of astroviruses. The origin of their name is the Greek word astron, which means star.
- They are non-enveloped RNA viruses with cubic capsids ranging in size from 28 to 35 nm and possessing T=3 symmetry.
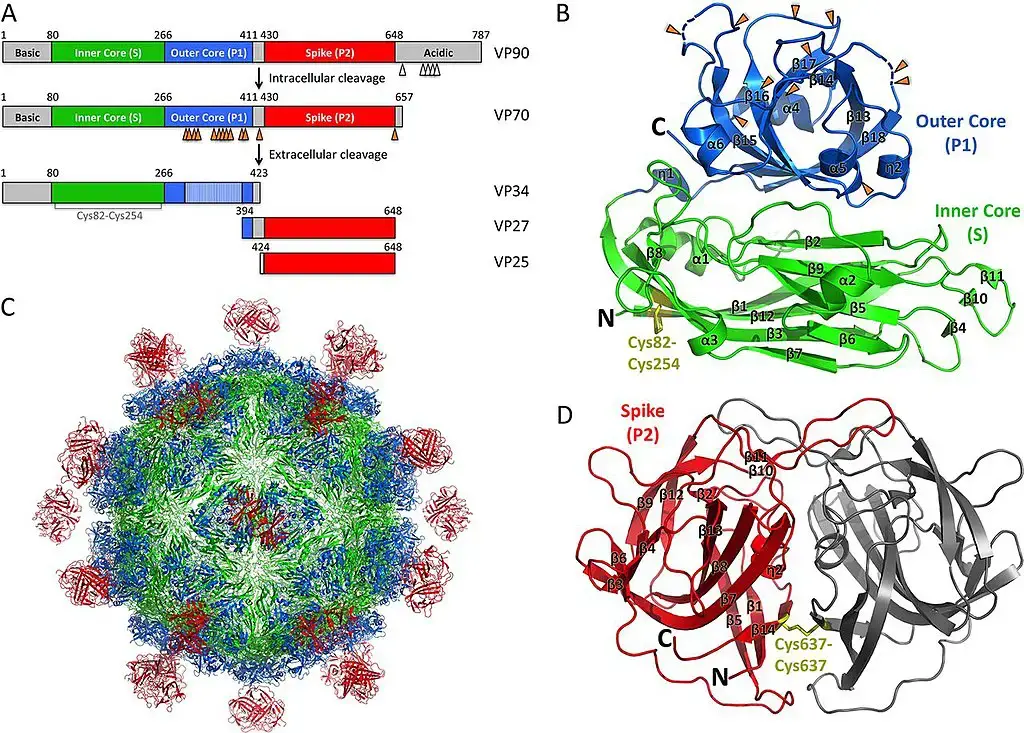
- Human astroviruses belong to the Mammastrovirus genus, which consists of eight serotypes. The capsid spikes of the human astrovirus have a characteristic structure.
- The spike domain has a 3-layered beta-sandwich fold and a 6-stranded beta-barrel structure at its centre. The centre of the beta-barrel is hydrophobic.
- The beta-sandwich with three layers is packed outside of the beta-barrel. The spike creates a dimer as well. Similar protein projections were discovered on the capsid of the hepatitis E virus and this unique structure.
- The projection domain of the human astrovirus has a polysaccharide receptor binding site. The amino acid sequence of the astrovirus capsid protein has little relationship with other known viral proteins, but the hepatitis E virus is the closest match.
Genome of Human Astrovirus
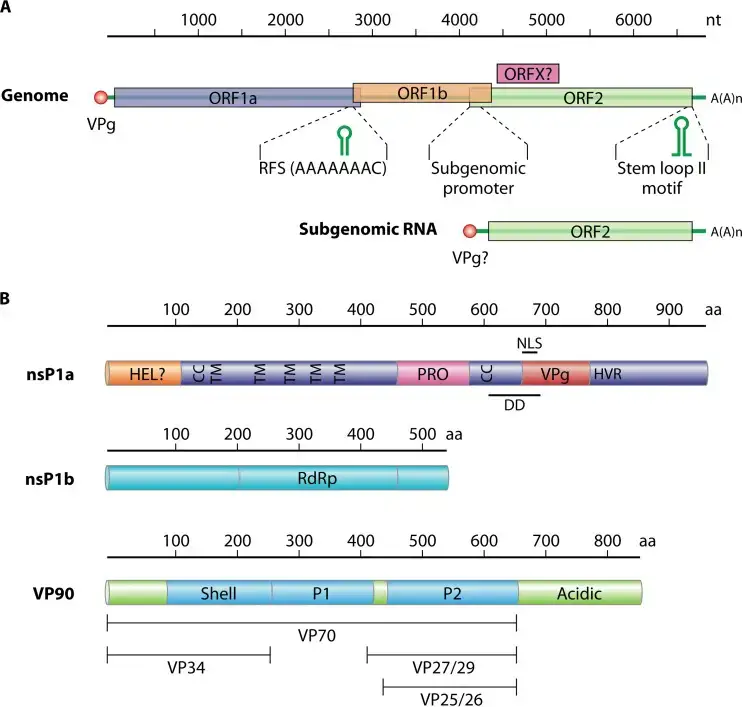
- The genome of an astrovirus is constituted of a single strand of positive sense RNA. The 3′ end of the strand has a poly A tail, but there is no 5′ cap.
- Without polyadenylation at the 3′ end, the genome is between 6.8 and 7.9 kilobases in length. The genome is divided into three open reading frames (ORFs), with a 70-nucleotide overlap between ORF1a and ORF1b.
- The remaining ORF is referred to as ORF2. [14] ORF2 encode the structural proteins, which include at least VP26, VP29, and VP32, with VP26 being the most antigenic and immunogenic.
- This protein is likely involved in the initial stages of viral infection, as it is a crucial component of the astrovirus life cycle.
- It has been estimated that the human astrovirus genome mutation rate is 3.7×10−3 nucleotide substitutions per site per year, whereas the synonymous change rate is 2.8×10−3 nucleotide substitutions per site per year.
- Strains of type-3 and type-4 human astroviruses, as well as porcine astroviruses, appear to possess the capacity for genetic recombination.
Epidemiology
- The most frequent method of transmission is assumed to be person-to-person fecal-oral dissemination. All eight HAstV serotypes or genotypes have been widely detected worldwide, with HAstV-1 being the most frequently found using both serotyping and genotyping, according to recent work with sensitive assay techniques that has revealed the prevalence of this virus to be much higher than previously thought.
- Children who have diarrhoea may pass astroviruses in their stools, though often at lower rates than rotavirus and norovirus. Most symptomatic infections affect the elderly and young children, Rates can reach 16% in studies conducted in hospitals and communities across several nations.
- In addition, a variety of locations, including schools, daycare facilities, and hospital wards, have been the site of outbreaks. Although outbreaks have been recorded in adult and geriatric populations, gastroenteritis is typically reported in young children.
- In temperate settings, some studies have found a higher incidence in the winter, although infection does not seem to be seasonal closer to the equator.
- Seroprevalence studies demonstrate that infection is widespread, and that children quickly develop antibodies. By the time they reach the age of nine, 94% of children in USA studies have antibodies to HAstV1 and 42% have antibodies to HAstV3; seroprevalence data indicate that people can contract more than one serotype.
Transmission and Tissue Tropism
- The most prevalent way that human astroviruses transmit is by fecal-oral contact, while improved community sanitation standards don’t seem to make a difference since infant infection rates are comparable in wealthy and underdeveloped nations.
- The most likely mode of transmission in outbreaks in daycare facilities and nursing homes is person-to-person contact. Contaminated water or foods, particularly shellfish, can potentially cause infection.
- The majority of astroviruses in humans and animals have been linked to gastroenteritis symptoms and have been shown to replicate in the digestive system.
- Duck hepatitis virus is a noteworthy exception, as it has been found by EM in the livers of animals exhibiting histological signs of hepatitis.
Replication of Human Astrovirus
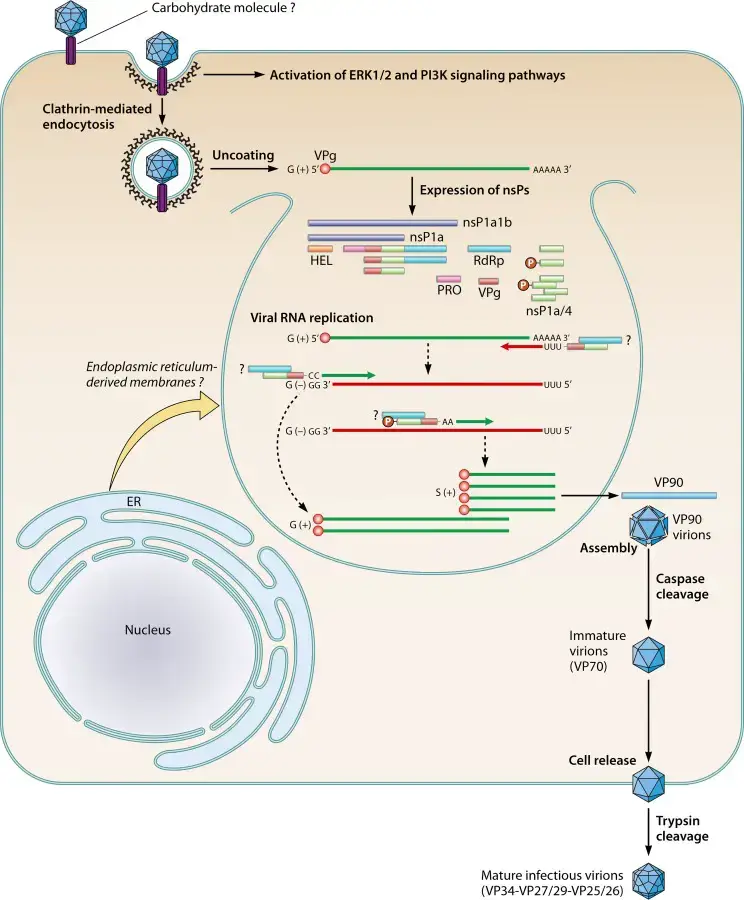
- Virus entry into the host cell is presumably mediated by attachment to host receptors.
- Uncoating followed by the release of viral genomic RNA into the cytoplasm.
- The genome is then translated, yielding nsP1a1b and nsP1a polyproteins, which are then cleaved by the viral serine protease (in nsP1a) and some cellular proteases, resulting in the nonstructural protein.
- Proteolytic processing of polyproteins nsP1a1b (about 160 kDa) generates the nsP1b protein (RdRp) (approximately 57 kDa) and the nsP1a protein (approximately 102 kDa), which is then cleaved to give many mature products.
- Replication happens in viral factories composed of ER-derived membrane vesicles.
- A full-length negative-sense genomic RNA would be generated and used as a template for the synthesis of positive-sense genomic RNA and subgenomic RNA.
- RNA translation at the subgenomic level generates the capsid protein precursor VP90.
- VP90 polyprotein initially assembles into immature virions in association with intracellular membranes via its C terminus, and several cellular caspases cleave these VP90 polyproteins near their C termini once they have dissociated from membranes, resulting in viral capsids composed of 70-kDa polyproteins (VP70).
- Finally, viral particles carrying VP70 would be liberated from cells and proteolytically digested by trypsin to increase their infectiousness.
- Release of the virus through cell lysis and maturation of the capsid through proteolytic cleavages.
Pathogenesis of Human Astrovirus
- The virus may spread from person to person via the fecal-oral route or through contaminated food or drink.
- Astrovirus pathogenesis has not been widely explored in humans.
- Viral particles have been observed by electron microscopy in intestinal epithelial cells and in epithelial cells located in the lower portion of the villi, indicating that the intestine is the location of viral replication; however, systemic spread has been reported in immunocompromised infants.
- Astroviruses cause gastroenteritis by destroying the intestinal epithelium, which inhibits the normal absorption mechanism, eliminates secretory activities, and decreases intestinal epithelial permeability.
- Mucosal IgA levels in the gastrointestinal tract have been demonstrated to be important immune effectors against gastrointestinal viral infections, and IgAs are induced by AstV infection; however, it is not yet known whether they are essential for protection.
- The presence of HAstV-specific CD4+ and CD8+ T cells in the normal tissue of intestinal biopsies from healthy adults in an organ culture system with inactivated HAstVs was demonstrated by the presence of HAstV-specific CD4+ and CD8+ T cells in intestinal biopsies from healthy adults.
- This demonstrates that both humoral and cellular adaptive immune responses have a role in protecting healthy people from reinfection.
- Stools can contain extremely high quantities of virus (up to 1013 genome copies/g), and virus excretion can be found for up to two weeks after symptoms have subsided.
Clinical Features and Infection
- In young children, astrovirus infection causes a moderate, self-limiting gastroenteritis with an incubation period of 2 to 4 days and a duration of 2 to 3 days.
- The infection of adult volunteers with human astroviruses resulted in diarrhoea and vomiting, as well as fever and stomach pain.
- Experimental infection with minimal levels of clinical illness, while sero-conversions in the majority of individuals can be measured. This finding implies that early infection may provide protection against symptomatic infections later in life.
Pathology and Histopathology
- The pathogenesis of astrovirus infection in humans is poorly understood. A single study shows the presence of astrovirus particles in the intestinal epithelial cells of two toddlers who shed virus in their faeces.
- During experimental infection of gnotobiotic lambs with OAstV, histologic alterations have been thoroughly reported. Astrovirus particles were detected in the apical two-thirds of villi columnar epithelial cells 14 hours after infection (PI).
- By 120 h PI, the villi had reverted to normal following the shedding of villus epithelial cells and the discharge of virus particles between 23 and 38 h PI. In contrast, experimental infection of gnotobiotic calves with BAstV resulted in subclinical infection. In the small intestine, virus particles were found in M cells covering Peyer’s patches.
Immune Response
- Primary infection with human astroviruses produces type-specific serum antibodies detectable by immunological EM, EIA, or neutralisation.
- As evaluated by neutralising antibody to HAstV types 1 to 7, the seroprevalence rates among 242 individuals in the Netherlands were 91, 31, 69, 56, 36, 16 and 10%, respectively.
- Antibody levels decreased in the elderly, which may explain the higher risk of symptomatic infection in this age group.
Diagnosis of Human Astrovirus
- Direct transmission electron microscopy (EM) is regularly used to detect HAstVs in negatively stained stool samples.
- Although some classic HAstVs can be propagated in cell culture with the use of trypsin, virus isolation in cell culture is not appropriate for astrovirus identification in faeces.
- Virus is captured using an enzyme immunoassay using a monoclonal antibody to the group antigen.
- The HAstV-1 specific latex agglutination test has been reported.
- Methods using ELISA and immunofluorescence to detect serum antibody.
- The genotyping of human astroviruses has been accomplished using RT-PCR.
Treatment of Human Astrovirus
Astrovirus Immunoglobulin
In a research by Bjorkholm et al., a 78-year-old patient with Waldenstrom’s macroglobulinemia was given 0.4 g/kg of astrovirus immunoglobulin for four days, and the patient’s symptoms disappeared, resulting in a full recovery from astrovirus. However, additional testing is required.
Achyrocline bogotensis antiviral therapy
In a study by Tellez et al., Achyrocline bogotensis plant extracts were employed to produce an antiviral treatment for both rotavirus and astrovirus. Achyrocline bogotensis was frequently utilised to treat cutaneous and urinary tract infections. Application of the extract to cells for pre-treatment (blocking), direct viral activity (proof of killing the virus), and therapy were components of the drug testing process (a decrease in the viral load after an infection is established). The extract exhibited direct antiviral efficacy by killing astroviruses directly and by reducing the viral load in patients with an established infection. During the experiment, there was no evidence of a pre-treatment effect.
Prevention and control of Human Astrovirus
- Since similar disease rates have been observed in developing and wealthy nations, it is unlikely that improvements in community sanitation will diminish the prevalence of astrovirus infection.
- During outbreaks at childcare centres or nursing homes, strict adherence to basic enteric infection control methods may help prevent the spread of infection.
- As primary infection with astrovirus appears to impart long-term immunity, as indicated by the low occurrence of symptomatic infection in adults, there is excellent potential for the development of a preventative vaccine.
References
- Monroe, S. S. (1999). Astroviruses (Astroviridae). Encyclopedia of Virology, 104–108. doi:10.1006/rwvi.1999.0017
- Kang, G., & Gray, J. J. (2013). Astroviruses. Hunter’s Tropical Medicine and Emerging Infectious Disease, 286–288. doi:10.1016/b978-1-4160-4390-4.00177-6
- Astroviridae. (2012). Virus Taxonomy, 953–959. doi:10.1016/b978-0-12-384684-6.00081-1
- Bosch A, Pintó RM, Guix S. Human astroviruses. Clin Microbiol Rev. 2014 Oct;27(4):1048-74. doi: 10.1128/CMR.00013-14. PMID: 25278582; PMCID: PMC4187635.
- Finkbeiner, S.R., Kirkwood, C.D. & Wang, D. Complete genome sequence of a highly divergent astrovirus isolated from a child with acute diarrhea. Virol J 5, 117 (2008). https://doi.org/10.1186/1743-422X-5-117
- https://www.annualreviews.org/doi/10.1146/annurev-virology-101416-041742
- https://www.atcc.org/products/vr-1936
- https://viralzone.expasy.org/27?outline=all_by_species