Hepatitis B is a viral infection that causes liver inflammation, sometimes leading to serious liver damage. The hepatitis B virus (HBV) is transmitted through contact with the blood or other body fluids of an infected person. This can occur through sexual contact, sharing needles, or from an infected mother to her baby at birth.
Symptoms of hepatitis B may include fever, fatigue, loss of appetite, nausea, vomiting, abdominal pain, dark urine, clay-colored bowel movements, joint pain, and yellowing of the skin and whites of the eyes (jaundice). These symptoms can range from mild to severe and may not appear for several weeks after exposure to the virus.
In some cases, people with hepatitis B do not have any symptoms and may not know they are infected. However, even if symptoms are not present, the virus can still cause liver damage and be transmitted to others.
There is a vaccine that can prevent hepatitis B infection. If you think you may have been exposed to the virus or are at high risk of infection, talk to your doctor about getting the vaccine.
What Is Hepatitis B?
Hepatitis B is the most prevalent severe liver illness worldwide. Hepatitis B is caused by a virus that infects and damages the liver. Two billion individuals, or one-third of the world’s population, have been infected with hepatitis B, and around 300 million live with a chronic infection. Despite the knowledge that hepatitis B is preventable and treated, it kills up to one million people every year.
The transmission of hepatitis B is through direct contact with infected blood or other bodily fluids. Due to the exchange of blood that occurs during birthing, the virus is most typically transferred from an infected pregnant woman to her unborn child. It is also transmitted by sharing personal goods such as razors, toothbrushes, nail clippers, and body jewellery.
Hepatitis B is a “silent pandemic” since the majority of newly infected and chronically infected individuals do not exhibit symptoms. Consequently, they can inadvertently transmit the virus to others and perpetuate the spread of hepatitis B in silence. People who are chronically infected but have no symptoms can develop catastrophic liver diseases such as cirrhosis or liver cancer despite the fact that their liver is being silently harmed.
Hepatitis B can be prevented and treated, which is excellent news. A simple blood test can be used to diagnose a hepatitis B infection. Testing is the only technique to definitively determine infection status. There is a safe vaccine for hepatitis B prevention. Chronic hepatitis B infections can be managed with excellent pharmacological regimens, and a cure is in reach.
Structure of Hepatitis B
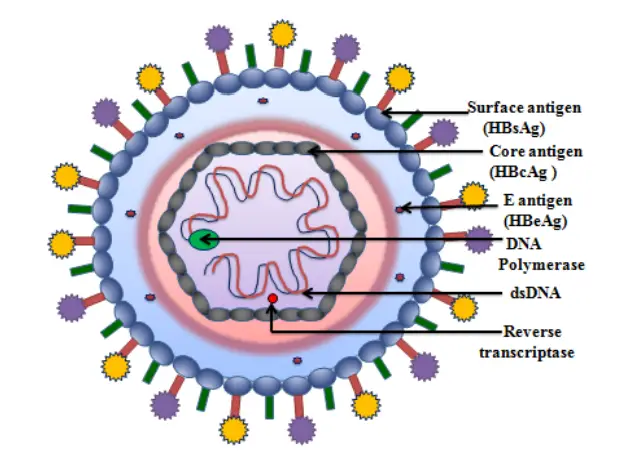
- Hepatitis B virus belongs to the family of Hepadnaviruses. The viral particle, also known as a Dane particle, is composed of an exterior lipid envelope and an icosahedral protein nucleocapsid core.
- The nucleocapsid encases viral DNA and a DNA polymerase with reverse transcriptase activity comparable to that of retroviruses.
- The outer envelope contains proteins that contribute to viral binding and penetration into vulnerable cells. With a virion diameter of 42 nm, the virus is one of the smallest enveloped animal viruses, yet pleomorphic forms exist, including filamentous and spherical bodies without a core.
- These non-infectious particles are made of the lipid and protein that form part of the virion’s surface, which is known as the surface antigen (HBsAg) and is produced in excess during the virus’s life cycle.
Components of Hepatitis B
It comprises:
- HBsAg (Hepatitis B surface antigen) was the first discovered hepatitis B viral protein. It’s made up of small (S), medium (M), and big (L) proteins.
- HBcAg (Hepatitis B core antigen) is the principal structural protein of the HBV icosahedral nucleocapsid, and it plays a role in viral replication.
- Capsid development is the primary factor in cell infection. It is unknown if HBcAg must be in the capsid form in order to contribute to viral clearance in vivo.
- Along with the pre-genomic RNA, Hepatitis B virus DNA polymerase is integrated into the nucleocapsid (pgRNA).
- The pgRNA undergoes reverse transcription within the capsid to produce the (-) DNA strand. The RNase activity of the polymerase simultaneously degrades the majority of the RNA template. After (+) DNA strand synthesis, the polymerase becomes covalently attached to the (-) DNA strand. After a virion has infected a new cell, the polymerase is discarded.
- HBeAg (Hepatitis B envelope antigen) is located between the icosahedral nucleocapsid core and the lipid envelope; nonetheless, it is nonparticulate, is secreted, and accumulates in serum. The HBeAg and HBcAg reading frames are identical.
- HBx is small, 154 amino acids in length, nonstructural, and plays a crucial role in HBV replication in HepG2 cells and HBV-associated liver disease.
- Numerous activities have been connected to HBx expression. However, the molecular processes behind the majority of these functions remain unclear. This multifunctional protein promotes cellular signalling pathways and is required for viral infection.
- Hepatitis D virus requires HBV envelope particles to become virulent.
Genome Organization of HBV
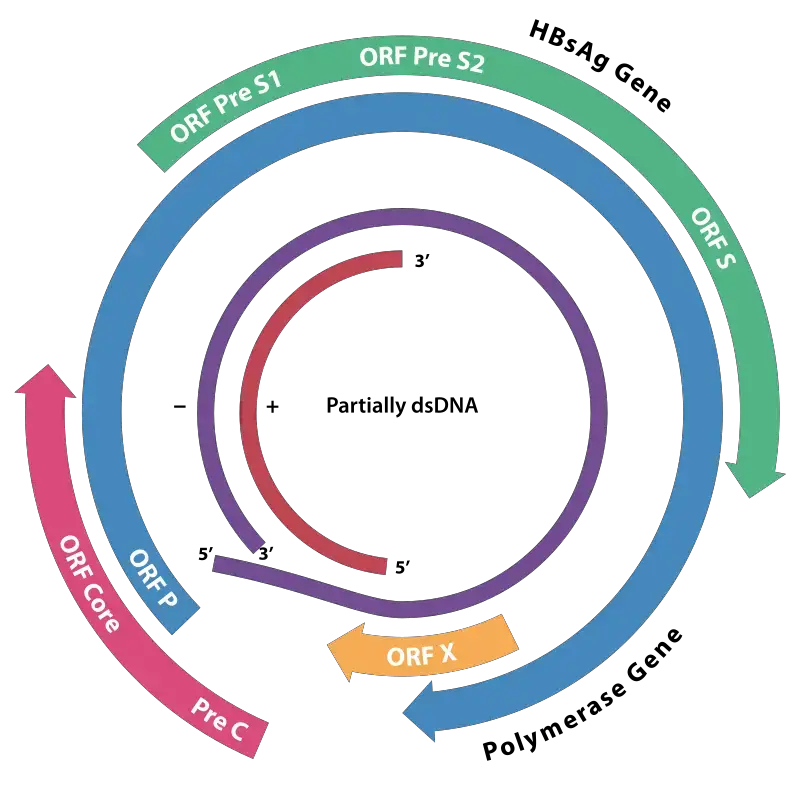
- The HBV genome is composed of circular DNA, however it is remarkable because the DNA is not double-stranded throughout. The viral DNA polymerase is attached to one end of the full-length strand.
- The genome consists of 3020–3320 nucleotides (for the full-length strand) and 1700–2800 nucleotides (for the shorter strand) (for the short length strand).
- The negative-sense, non-coding (non-coding) strand is complementary to the viral mRNA. Immediately upon infection, the viral DNA is discovered in the nucleus.
- The partially double-stranded DNA becomes fully double-stranded through the completion of the (+) sense strand by cellular DNA polymerases (viral DNA polymerase is used at a later stage) and the elimination of the viral polymerase protein (P) from the (-) sense strand and a short sequence of RNA from the (+) sense strand. Non-coding nucleotides are deleted from the ends of the (-)sense strand before rejoining the ends.
- Covalently closed circular DNA (cccDNA) serves as a template for the transcription of viral genes in the nucleus of the host cell by RNA polymerase II.
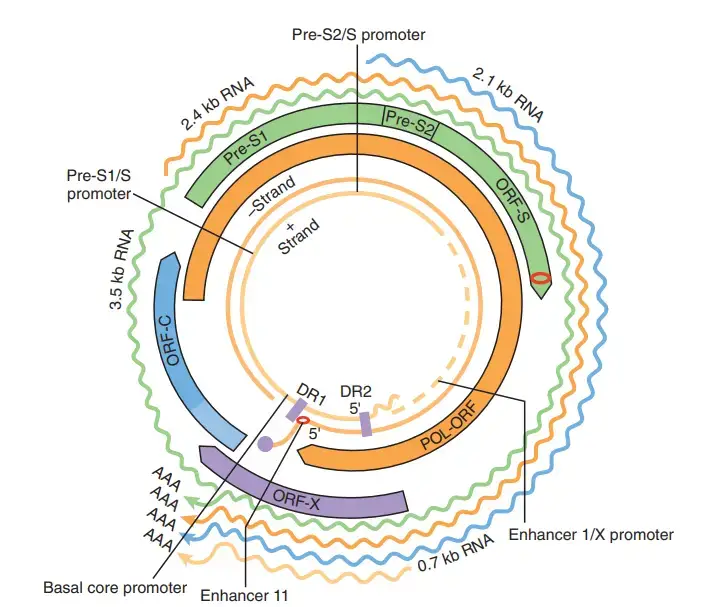
- In the HBV genome, two enhancers named enhancer I (EnhI) and enhancer II (EnhII) have been found. Both enhancers are more active in cells of hepatic origin, and collectively they control the expression of the entire viral transcripts.
- C, P, S, and X are the four known genes that the genome encodes. Gene C (HBcAg) encodes the core protein, and its start codon is preceded by an upstream in-frame AUG start codon from which the pre-core protein is created.
- The pre-core protein is processed proteolytically to generate HBeAg. Gene P codes for the DNA polymerase. Gene S is the gene that encodes the surface antigen (HBsAg).
- The HBsAg gene is one continuous open reading frame, but it contains three “start” (ATG) codons that divide it into three portions, pre-S1, pre-S2, and S. Due to the presence of numerous start codons, polypeptides of three distinct sizes are produced: large, middle, and tiny (pre-S1 + pre-S2 + S, pre-S2 + S, or S).
- Some evidence suggests that the gene X-encoded protein may function as a transcriptional transactivator, although its function is not completely understood. Intriguingly, a 40 kDa X-Core fusion protein is encoded by a lengthy, 3.9 kb viral transcript whose function is unknown.
- After the second round of transcription, the 3.9 kb RNA is polyadenylated. The synthesis of the 3.9 kb RNA begins at the promoter region of the X gene. Other long pregenomic/pre-core (pg/pc) RNA species have identical characteristics. The viral transcription machinery must therefore disregard the poly(A) signal during the initial transcription round.
- Multiple noncoding RNA elements have been discovered in the HBV genome. Included among these are HBV PREalpha, HBV PREbeta, and HBV RNA encapsulation signal epsilon.
Life cycle of HBV
The Hepatitis B virus has a complex life cycle. Hepatitis B is one of the few non-retroviral viruses known to utilise reverse transcription in its replication process.
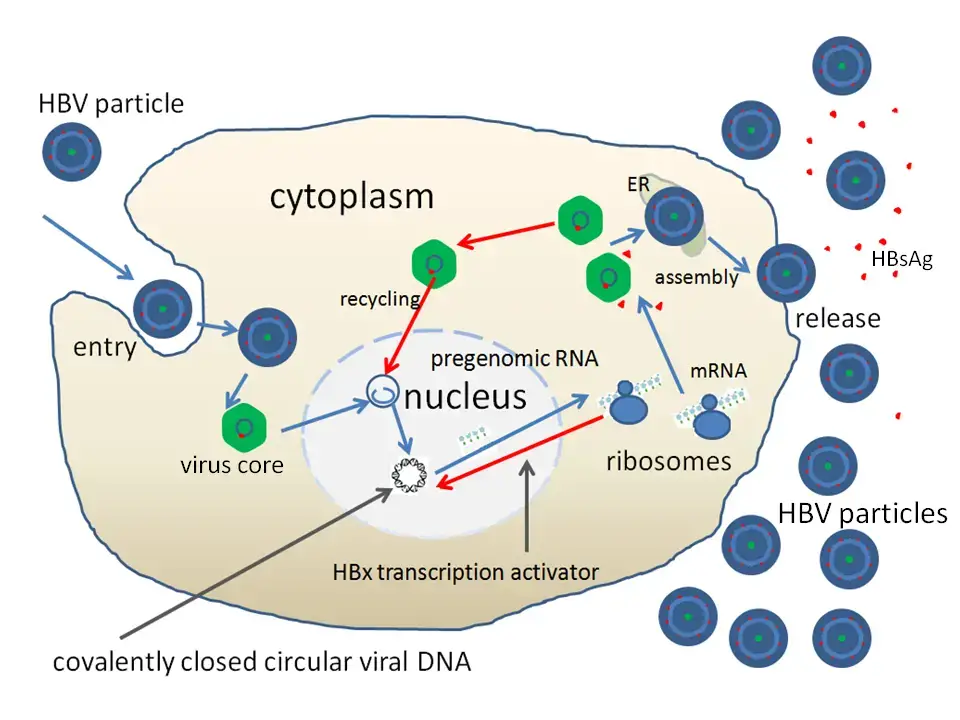
1. Attachment
- By attaching to receptors on the surface of the cell and entering through endocytosis mediated by either clathrin or caveolin-1, the virus gains access to the cell.
- HBV attaches to heparin sulphate proteoglycan first. The pre-S1 region of the HBV L protein binds firmly to the SLC10A1gene-encoded cell surface receptor sodium taurocolate cotransporting polypeptide (NTCP).
- The majority of NTCP is located in the sinusoidal membrane of liver cells. The tissue specificity of HBV infection correlates with the presence of NTCP in liver cells.
2. Penetration
- After endocytosis, the membrane of the virus fuses with the membrane of the host cell, releasing the nucleocapsid into the cytoplasm.
3. Uncoating
- Because the virus replicates through the production of RNA by a host enzyme, the viral genomic DNA must be transported to the nucleus of the host cell.
- Microtubules are believed to transfer the capsid to the nuclear pore. The core proteins separate from the partly double-stranded viral DNA, which is then converted into covalently closed circular DNA (cccDNA), which acts as a template for transcription of four viral mRNAs.
4. Replication
- The biggest mRNA (which is longer than the viral genome) is utilised to produce new copies of the viral genome as well as the capsid core protein and viral RNA-dependent-DNA-polymerase.
5. Assembly
- These four viral transcripts undergo additional processing and form progeny virions, which are either discharged from the cell or returned to the nucleus to be re-cycled.
6. Release
- The lengthy mRNA is subsequently carried back to the cytoplasm, where the reverse transcriptase activity of the virion P protein synthesises DNA.
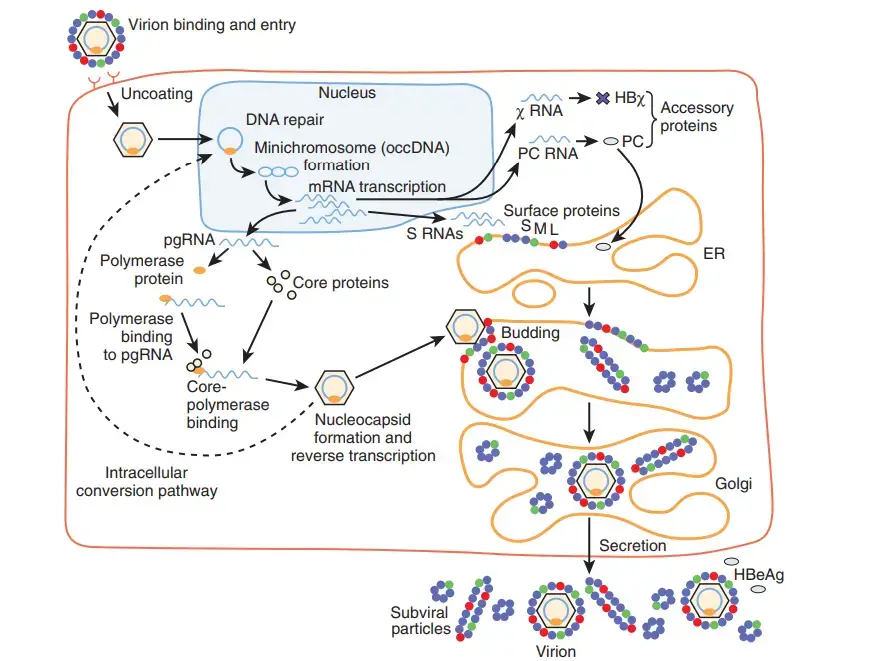
HBV replication strategy
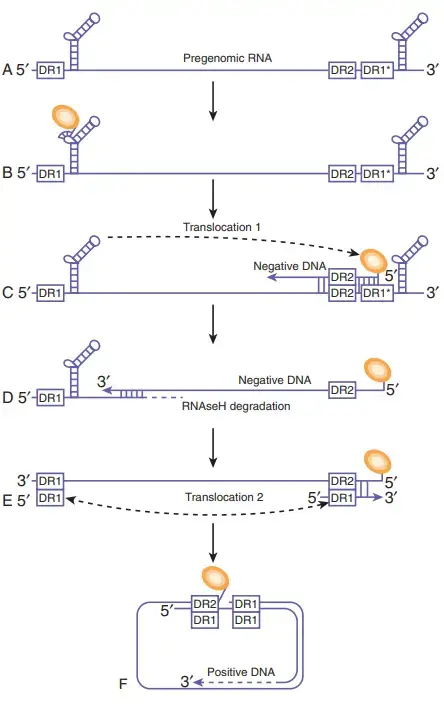
- The blue line represents pgRNAs longer than the genome. As hairpin structures, the identical -sequences at the 5′ and 3′ ends of the genome are depicted. The direct repeat items are displayed as rectangular boxes. An asterisk denotes the second copy of DR1 at the 3′ end of the genome.
- The polymerase protein (orange circle) attaches to the 5′ ε and then synthesises a short oligonucleotide utilising the polymerase’s terminal protein as a primer and the bulge region of ε as a template.
- This complex is encapsulated by core protein dimers to form the nucleocapsid (not shown). The polymeraseoligonucleotide complex translocates to DR1 to commence reverse transcription of the HBV genome’s negative strand. Except for the 5′ DR1 sequence, the RNA template is destroyed by the RNAseH activity of the HBV polymerase.
- The 18-nucleotide RNA sequence is subsequently transmitted to DR2 and employed as a primer for positive-strand synthesis. Genome circularization is possible due to an 8-nt terminal redundancy.
Transmission of Hepatitis B
- In highly endemic regions, hepatitis B is typically transmitted from mother to child at birth (perinatal transmission) or through horizontal transmission (exposure to infected blood), particularly from an infected child to an uninfected child during the first five years of life. It is typical for infants infected from their mothers or before the age of 5 to acquire a persistent infection.
- Hepatitis B is also transmitted through needlestick injuries, tattooing, piercing, and exposure to infected blood and body fluids, including saliva, menstrual, vaginal, and seminal secretions. The virus can also be transmitted by the reuse of contaminated needles, syringes, or other sharp objects in health care settings, the community, or among drug users. Sexual transmission is more common among unvaccinated individuals with several partners.
- In less than 5% of cases, Hepatitis B infection acquired in maturity results in chronic hepatitis, whereas infection in infancy and early childhood results in chronic hepatitis in approximately 95% of cases. This is the foundation for enhancing and prioritising baby and kid vaccinations.
- The hepatitis B virus can persist for at least seven days outside the body. During this period, the virus can still cause infection if it enters the body of an unvaccinated individual. The hepatitis B virus has an incubation period ranging from 30 to 180 days. The virus can be diagnosed 30 to 60 days after infection and can persist and develop into chronic hepatitis B, particularly when transmitted during infancy or youth.
Pathogenesis of Hepatitis B
- There appear to be three pathways involved in liver cell damage during HBV infections.
- The initial reaction is an HLA class I-restricted cytotoxic T-cell (CTL) response against HBcAg/HBeAg on HBV-infected hepatocytes.
- The direct cytopathic effect of HBcAg expression in infected hepatocytes is a second potential mechanism.
- A third proposed mechanism involves a high amount of HBsAg expression and ineffective secretion.
- In the acute phase, the portal tracts exhibit symptoms of inflammation, and the infiltration is mostly lymphocytic. As they die, infected hepatocytes in the liver parenchyma exhibit ballooning and produce acidophilic (Councilman) bodies.
- In chronic hepatitis, damage extends beyond the portal tracts, creating the appearance of piecemeal necrosis. There is also some lobular inflammation present. As the condition advances, fibrosis and ultimately cirrhosis occur.
- Continued immune-mediated death of hepatocytes expressing viral antigens causes chronic liver injury. In addition, autoimmune reactions may contribute to the damage, as certain liver-specific antigens induce immune responses.
Diagnosis of Hepatitis B
The goal of diagnostic laboratory tests of patients with clinical hepatitis is to, first, identify the causative hepatitis virus and, second (for HBV), differentiate between acute and chronic infections. Hepatitis is diagnosed based on clinical symptoms and biochemical testing that detect liver damage. Aminotransferases, bilirubin, and prothrombin time elevations all contribute to the initial diagnosis of hepatitis. ELISA, also known as enzyme-linked immunosorbent assay, and other immunologic techniques for detecting viral antigens and antibodies are the principal way of differentiating between HAV, HBV, HCV, and HDV. In addition, the presence or lack of particular antiviral antibodies and viral antigens enables the distinction between acute and chronic HBV infections.
- The most common method for diagnosing HBV is the detection of HBsAg in the bloodstream. Other routinely conducted tests include HBeAg, HBV DNA, and anti– core antibody.
- IgM anti–core antibody is symptomatic of recent HBV exposure, whereas IgG core antibody is present in persons with chronic infection and those who have recovered from infection. Subjects who have been effectively inoculated with HBV vaccine are positive for anti-HBsAg.
- If a liver biopsy specimen is available, immunohistochemistry using an antibody against the surface antigen can be conducted.
- Screening has been suggested for all pregnant women and newly arriving immigrants from countries with an HBV prevalence rate of at least 2%.
- Current research investigates whether pregnant women living in regions with high HBV endemicity should be screened for occult HBV infection utilising sensitive assays for HBV DNA.
Clinical significance of Hepatitis B
Clinical significance: acute disease
HBV is significant to medicine and public health not only as the cause of acute liver disease, but also as the source of chronic, persistent infections that can lead to the death of infected individuals from cirrhosis and liver cancer. Chronically infected individuals act as the virus reservoir in the population. In the majority of cases, the original infection is asymptomatic and disappears due to an efficient cell-mediated immune response.
1. Phases in acute hepatitis B virus infections
- Following infection, the incubation period for HBV varies between 45 and 120 days. This is followed by a pre-icteric (prejaundice) phase that lasts several days to a week. Symptoms include moderate fever, lethargy, anorexia, myalgia, and nausea.
- The acute, icteric phase then lasts between one and two months. During this period, bilirubinuria and jaundice (a yellowish hue of the mucous membranes, conjunctivae, and skin) are apparent.
- Typically, there is also an enlarged and painful liver. In 80 to 90 percent of people, many more months of convalescence are followed by full recovery.
2. Monitoring the course of acute hepatitis B virus infection
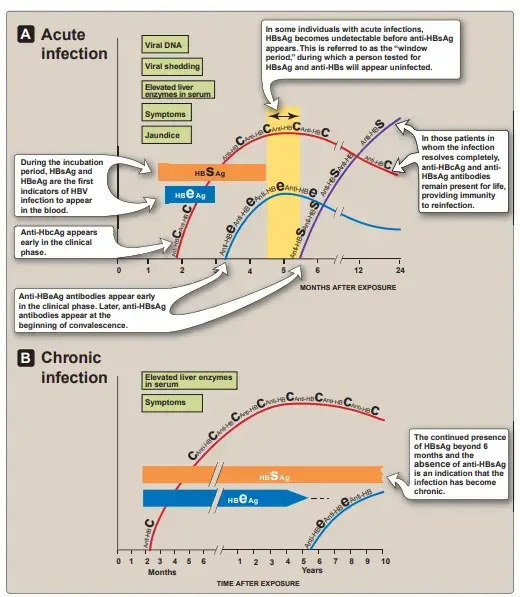
- HBV infection is unique in that the quantities of virions and virion components in the blood are so high that the time course of their appearance and clearance, as well as that of the antibodies directed against them, serve as convenient markers of the stage of the disease and its likely future course.
a. Appearance of viral antigens
HBsAg and hepatitis B e antigen (HBeAg) are the first indications of HBV infection to show in the blood during the incubation phase. Their presence implies a current infection, although neither acute nor chronic infections are distinguished. Next, full virions, viral DNA, and viral DNA polymerase become detectable. During the acute disease phase, when a patient’s blood contains the highest titer of infectious virus, these continue to increase.
b. Appearance of antiviral antibodies
Antibodies to HBcAg rise alongside liver enzymes in the serum, whereas antiHBeAg and anti-HBs antibodies do not develop until the onset of convalescence (generally after the respective antigens have disappeared from the blood. In patients whose illness completely cures, anti-HBcAg and anti-HBsAg antibodies are persistent for life and provide immunity against reinfection. Chronic infection is indicated by the presence of HBsAg for more than six months and the absence of anti-HBsAg antibodies (Figure 26.8). Chronic HBV infection is capable of producing an immunological response against HBsAg, but anti-HBs antibody levels are undetectable. Every antibody produced is complexed with circulating HBsAg.
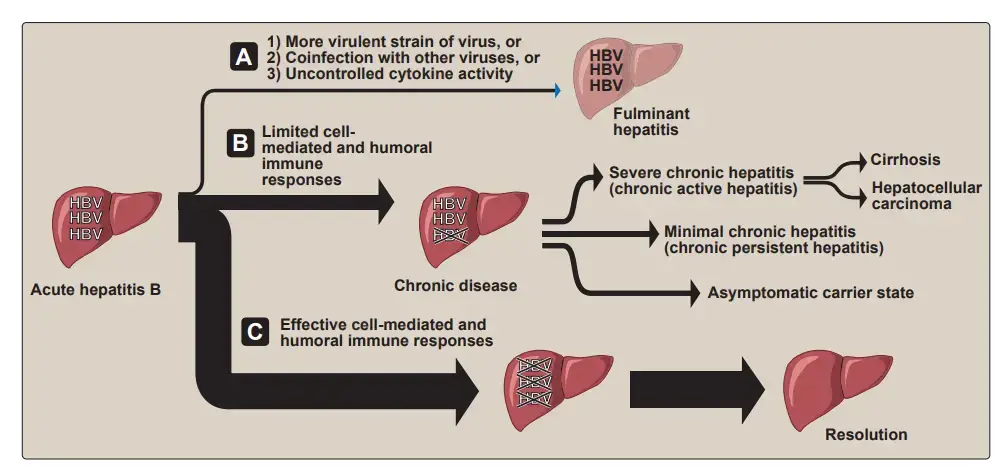
3. Fulminant hepatitis
In 1% to 2% of acute symptomatic patients, more widespread liver necrosis occurs within the first 8 weeks of the acute disease. This is accompanied by a high fever, stomach pain, and ultimately renal failure, coma, and seizures. This condition, known as fulminant hepatitis, is deadly in approximately 8 percent of cases. A highly virulent strain of HBV, coinfection with HDV or another hepatitis virus (such as HCV), and/or an unregulated immune response on the part of the patient are believed to play a role in the progression of acute hepatitis.
Clinical significance: chronic disease
In around two-thirds of people, the initial infection is asymptomatic, despite the fact that such patients may eventually develop symptomatic chronic liver disease, showing the virus’s persistence. Approximately 2 to 10 percent of adults and over 25 percent of young children are chronically infected following clearance of acute disease (or silent infection). The elevated rate of progression to chronic liver disease observed in infants born to HBV-infected mothers is believed to be attributable to the immaturity of newborns’ immune systems. Additionally, those with immunological deficits are significantly more likely to get persistent infections than those with normal immune systems.
1. Types of chronic carriers
Asymptomatic HBsAg carriers are the most prevalent type of persistently infected persons. Typically, they have anti-HBeAg antibodies and little or no virus in their blood (see Figures 26.7B and 26.8). In such patients, development of liver damage or recurrence of acute hepatitis episodes is uncommon. Carriers with mild chronic hepatitis (formerly known as “chronic persistent hepatitis”) are asymptomatic the majority of the time, but have an increased risk of disease reactivation, and a tiny percentage proceed to cirrhosis. Severe chronic hepatitis (formerly known as “chronic active hepatitis”) is characterised by more frequent exacerbations of acute symptoms, such as progressive liver damage that may develop to cirrhosis and/or hepatocellular cancer (see below), persistent fatigue, anorexia, malaise, and anxiety. Active virus replication and the presence of HBeAg in the blood are associated with these symptoms. In varied degrees, serum levels of liver enzymes and bilirubin are elevated, suggesting the severity of necrosis. Carriers with more frequent recurrences of acute illness and those in whom HBeAg is not eliminated from the blood, indicating ongoing viral replication, have the highest risk of developing cirrhosis. The overall life expectancy of persons with cirrhosis is severely reduced.
2. Development of hepatocellular carcinoma (hepatoma)
In regions with high HBV endemicity, the incidence of hepatocellular carcinoma (HCC) is 10 to 100 times higher than in the United States. In all groups, males had a higher incidence of chronic HBV infections, a higher incidence of progression to cirrhosis, and a higher incidence of HCC, with a male-to-female ratio of 6:1. HCC often manifests many years after the initial HBV infection, and the tumour itself grows slowly and metastasizes seldom. A patient with HCC manifests clinically with weight loss, right upper quadrant pain, fever, and intestinal haemorrhage. Although persistent HBV infection significantly raises the risk of HCC, the mechanisms between HBV and HCC are not well understood. Chronic HBV infection gives the chance for chromosomal rearrangements and mutations by generating recurrent liver necrosis followed by regeneration of the damaged tissue. Due to the fact that HBV is a DNA virus, integration of the viral genome into the host’s chromosome can also lead to mutation and insertion, as well as alterations in cell growth control. After integration of the gene into the host’s chromosome, current data suggests that the HBV gene product X is actively implicated in tumour formation. HCC is a leading cause of cancer-related mortality worldwide, and its prevalence tracks HBV incidence (approximately 80 percent of primary HCCs occur in HBV-infected individuals).
Treatment of Hepatitis B
Acute hepatitis
Acute hepatitis B typically does not require specific treatment since, in approximately 95% of individuals, the immune system controls the infection and destroys the virus within six months. Despite the fact that pharmacological therapy is typically only required for chronic hepatitis, it may also be necessary for acute severe liver impairment caused by fulminant hepatitis.
Chronic hepatitis
The objective of treatment for chronic hepatitis is to lower the risk of progressive chronic liver disease and other long-term consequences of chronic HBV, such as cirrhosis and hepatocellular cancer. Interferon- or one of a vast range of nucleoside/nucleotide antiviral medicines are among the most frequently used antiviral medications. Multiple factors, including the patient’s antibody and antigen status, influence the medicine of choice. Pegylated interferon- (assuming the patient does not have cirrhosis), entecavir, or tenofovir are frequently the first-line treatments of choice. Seroconversion to anti-HBeAg and prolonged suppression of HBV DNA are the two most often utilised markers to monitor the success of therapy.
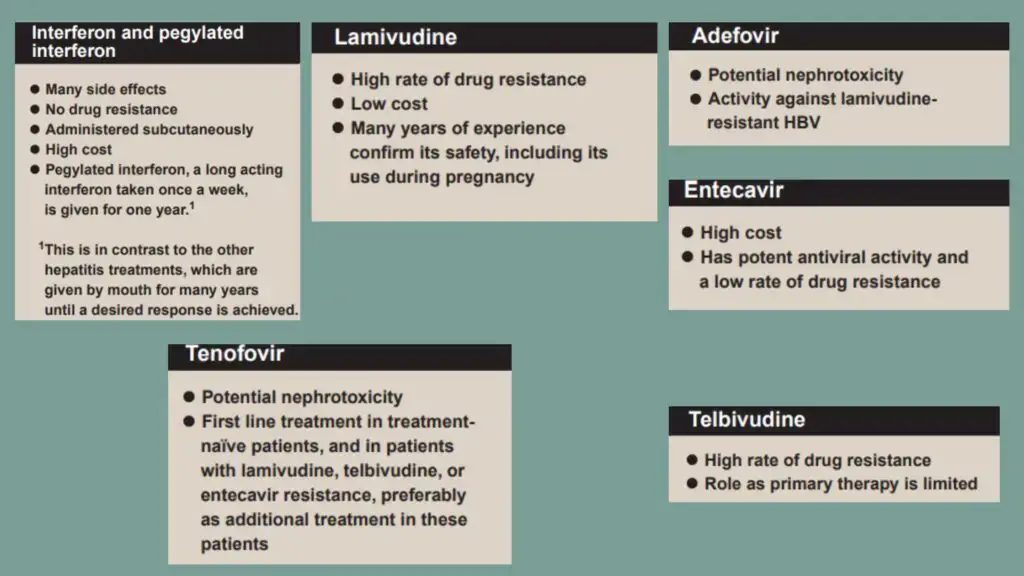
- The purpose of chronic hepatitis B treatment is to inhibit HBV replication, halt the course of liver disease, and so prevent the onset of cirrhosis, liver failure, and HCC.
- Interferon alpha (α-IFN) is the recommended first-line therapy for patients without cirrhosis. Interferon possesses antiviral, antiproliferative, and immunomodulatory properties.
- At a daily dose of 100 mg, lamivudine reduces or eliminates detectable HBV DNA in plasma in around 40% of HBeAg-positive patients and 60%–70% of HBeAg-negative patients.
- Telbuvidine is an analogue of L-nucleoside that has significant anti-HBV action. Similar in method of action and resistance profile to lamivudine, but more effective. Due to a high rate of resistance and cross-resistance with lamivudine, its use is restricted.
- Emtricitabine is another analogue of L-nucleosides with similar action to lamivudine.
- Adefovir is a nucleotide analogue of deoxyadenosine monophosphate that has shown efficacy in inhibiting HBV DNA by 20–50% and regulating liver function by 50–70%.
- The antiviral agent entecavir is a carbocyclic analogue of 2′-deoxyguanosine. It is a strong HBV replication suppressor, resulting in elimination of serum HBV DNA in 70–90% of patients and normalisation of ALT in 70–80% of patients.
- An analogue of nucleotides, tenofovir was initially licenced for the treatment of HIV. It is frequently utilised in combination with emtricitabine. After 48 weeks of treatment with tenofovir, 80–90% of patients achieve HBV DNA reduction and 70–80% of patients achieve ALT normalisation.
- Orthotopic liver transplantation is a treatment for end-stage liver damage caused by chronic hepatitis B. However, the probability of reinfection with HBV, probably through extrahepatic reservoirs in the body, is at least 80%.
Vaccines of Hepatitis B
- Hepatitis B is preventable by vaccination.
- Since 1982, a vaccination for hepatitis B has been available.
- The Hepatitis B vaccine is safe and effective and is typically administered as 3 to 4 injections over a period of 6 months.
- Plasma-derived vaccines: Purifying HBsAg coupled with 22-nm particles from healthy HBsAg-positive carriers and treating the particles with virus-inactivating chemicals yielded the first vaccination (formalin, urea, heat).
- Recombinant DNA-derived vaccines: Vaccines developed from recombinant DNA contain HBsAg generated by recombinant DNA in yeast cells or continuous mammalian cell lines. Although the polypeptide antigen produced by recombinant yeast is not glycosylated, the HBsAg synthesised in yeast produces particles of 15–30 nm in diameter that exhibit the morphology of free surface antigen in plasma. The vaccine prepared with this purified material has the same potency as vaccines containing antigen obtained from plasma.
- The HBsAg vaccinations (HB) may be combined with other vaccines, including Calmette-Guérin bacillus (BCG), measles, mumps, and rubella (MMR), Haemophilus influenzae b (Hib), and diphtheria, tetanus, and pertussis mixed with polio (DTP-polio).
Prevention and Control of Hepatitis B
The goal of preventing the spread of HBV infection is to reduce the incidence of acute hepatitis. A secondary objective is to reduce the number of chronically infected persons who serve as reservoirs for infectious virus in the population and are at a high risk of developing cirrhosis and liver cancer. The availability of a very potent vaccine has prompted a multifaceted strategy: 1) protection of adults at risk due to lifestyle or occupation; 2) protection of newborns from infection by transmission from HBV-positive mothers (important due to the high rate of resulting chronic infections; 3) protection of siblings and other children from infection by chronically infected family members.
Active immunization
- HBsAg is utilised in the preparation of protective vaccinations because antibody to the virion component neutralises infectiousness. H
- BV vaccination is currently recommended as a standard immunisation for neonates, as well as for teenagers who were not immunised as infants.
- An uncommon aspect of the suggested vaccination schedule is the birthtime initiation of an HBV vaccine series.
- This is achievable because newborns have a sufficient antibody response to neonatal HBV immunisation.
Passive immunization
- Hepatitis B immunoglobulin (HBIG) is manufactured from the blood of donors with a high anti-HBsAg antibody titer. Individuals mistakenly exposed to HBV-infected blood through a needlestick or other means, as well as those exposed to infection through sexual contact with an HBV-positive partner, should get HBIG immediately as the first step in preventing infection.
- In such instances, active immunisation with the hepatitis B vaccine should be administered. It is also highly suggested that pregnant women get HBsAg testing.
- Babies born to HBV-positive mothers are administered HBIG plus hepatitis B vaccine at birth, followed by further doses at 1 and 6 months.
References
- Singh, Jagtar & Sinha, Shweta. (2015). Volume-6, Issue-3, July-Sept-2015 Coden IJABFP-CAS-USA st st Received: 21 May-2015 Revised: 31 May-2015. International Journal of Applied Biology and Pharmaceutical Technology. 6. 80- 92.
- MacLachlan JH, Cowie BC. Hepatitis B virus epidemiology. Cold Spring Harb Perspect Med. 2015 May 1;5(5):a021410. doi: 10.1101/cshperspect.a021410. PMID: 25934461; PMCID: PMC4448582.
- Rifkind, D., & Freeman, G. L. (2005). HEPATITIS B VIRUS. The Nobel Prize Winning Discoveries in Infectious Diseases, 95–101. doi:10.1016/b978-012369353-2/50021-7
- Chan, H. L.-Y., & Wong, V. W.-S. (2012). Hepatitis B. Zakim and Boyer’s Hepatology, 540–563. doi:10.1016/b978-1-4377-0881-3.00030-9
- Karnsakul, W., & Schwarz, K. B. (2011). Hepatitis. Infectious Diseases of the Fetus and Newborn, 800–813. doi:10.1016/b978-1-4160-6400-8.00025-0
- Warner, N., & Locarnini, S. (2012). Replication of Hepatitis B Virus. Zakim and Boyer’s Hepatology, 86–96. doi:10.1016/b978-1-4377-0881-3.00006-1
- https://labpedia.net/hepatitis-b-virus-hbv-diagnosis-and-treatment/#Structure_of_Hepatitis_B_Virus_HBV
- https://www.news-medical.net/health/Hepatitis-B-Structure-Capsid-Flexibility-and-Function.aspx
- https://microbiologyinfo.com/hepatitis-b-virus-structure-epidemiology-symptoms-pathogenesis-diagnosis-treatment-and-vaccines/
- https://www.onlinebiologynotes.com/hepatitis-b-replication-transmission-pathogenesis-disease-diagnosis-and-treatment/
- https://www.who.int/news-room/fact-sheets/detail/hepatitis-b#:~:text=Hepatitis%20B%20is%20a%20potentially,from%20cirrhosis%20and%20liver%20cancer.
- https://www.cdc.gov/hepatitis/hbv/index.htm
- https://www.hepb.org/what-is-hepatitis-b/what-is-hepb/