What is Helicobacter pylori?
- Helicobacter pylori is a gram-negative, spiral-shaped bacterium that is commonly found in the stomachs of humans. It is one of the most prevalent bacterial infections worldwide, with an estimated two-thirds of the global population carrying the bacterium. However, the majority of these individuals are asymptomatic. Only certain strains of H. pylori are pathogenic, leading to a variety of gastric and extragastric disorders.
- The bacterium’s unique helical shape, which allows it to penetrate the thick mucosal lining of the stomach, is key to its ability to cause infection. The flagella, tail-like structures that propel the bacterium, help it move through the stomach’s viscous mucus layer. Once it reaches the less acidic mucosal lining, H. pylori can colonize the area. The bacteria are microaerophilic, meaning they require lower oxygen levels than those found in the atmosphere, which makes the stomach an ideal environment for survival.
- Infection with H. pylori is closely associated with various gastric conditions. The most common initial effect is gastritis, which is inflammation of the stomach lining. If the infection persists, it can progress to chronic gastritis, and in severe cases, it may lead to the formation of gastric ulcers. Prolonged inflammation and damage to the stomach lining increase the risk of developing gastric cancer. H. pylori is classified as a class 1 carcinogen, responsible for approximately 89% of all gastric cancer cases worldwide. Additionally, the bacterium is linked to other cancers, such as gastric MALT lymphoma.
- Beyond gastric disorders, H. pylori infection can lead to extragastric complications, including anemia, which may be due to either iron deficiency or vitamin B12 deficiency. Studies have also suggested associations with diabetes mellitus, cardiovascular disease, and neurological conditions. Interestingly, there is some evidence to suggest that H. pylori infection may have protective effects in certain conditions, such as asthma and inflammatory bowel diseases, though these findings are still under investigation.
- The pathogenesis of H. pylori is influenced by its array of virulence factors. The bacterium produces enzymes like urease, catalase, and oxidase, which help it survive in the harsh acidic environment of the stomach. Urease, in particular, is crucial because it neutralizes stomach acid, allowing the bacterium to thrive. H. pylori’s outer membrane contains various proteins, including adhesins, which allow the bacterium to adhere to the gastric epithelium, and porins, which help it transport nutrients.
- Treatment of H. pylori infection is possible through the use of antibiotics and proton-pump inhibitors, which have significantly reduced the prevalence of the infection in many developed countries. However, H. pylori remains more common in developing regions, where access to medical treatment may be limited.
Scientific classification of Helicobacter pylori
| Domain: | Bacteria |
| Phylum: | Campylobacterota |
| Class: | “Campylobacteria” |
| Order: | Campylobacterales |
| Family: | Helicobacteraceae |
| Genus: | Helicobacter |
| Species: | H. pylori |
Morphology of Helicobacter pylori
Helicobacter pylori is a Gram-negative bacterium with distinctive features that make it highly adapted to its environment. Its unique structure plays a critical role in its mobility and survival.
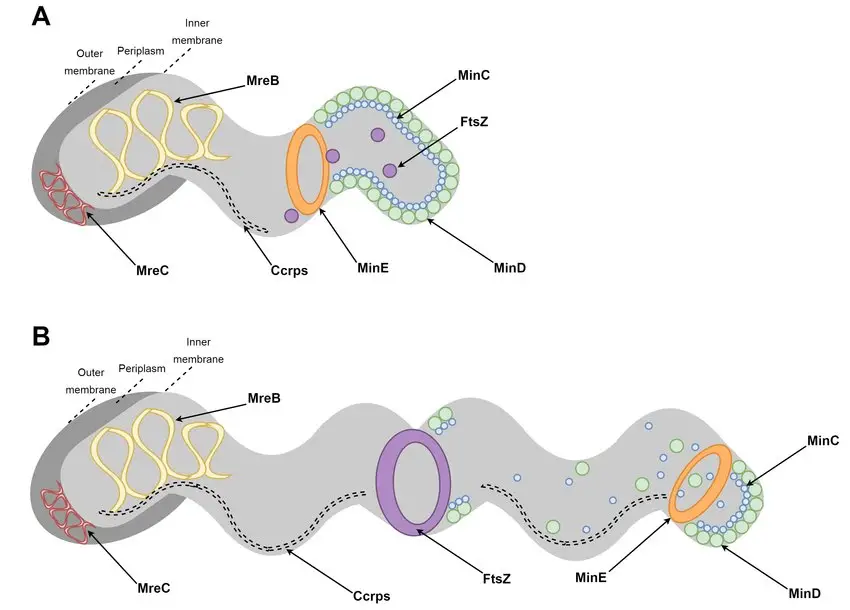
- Shape and Size:
The bacterium has a curved, spiral, or S-shaped structure.
It measures approximately 3 micrometers in length and 0.5–0.9 micrometers in width. - Motility Features:
Known for its exceptional motility, H. pylori exhibits corkscrew-like movement.
This is facilitated by a unipolar tuft of lophotrichous flagella, which drives its motion. - Non-Sporulating:
It does not form spores, which differentiates it from many other bacteria in terms of survival strategies.
These characteristics highlight the bacterium’s unique adaptations to colonize and persist in its specific habitat.
Genome of H. pylori Infection
The genome of Helicobacter pylori reveals a complex and diverse structure that helps explain its ability to adapt and persist in the human stomach. With a variety of strains sequenced, researchers have uncovered significant details about its genetic makeup and the mechanisms behind its survival.
- Diverse strains, diverse genomes: H. pylori is made up of numerous strains, each with its own unique genetic characteristics.
- Genome size: The genome of strain 26695 is approximately 1.7 million base pairs long, containing around 1,576 genes.
- Pan-genome: A larger picture emerges when considering the combined genomes of 30 sequenced strains. This “pan-genome” encodes 2,239 protein families, known as orthologous groups (OGs).
- Core and accessory genomes: Of the 2,239 OGs, 1,248 are present in all 30 strains, forming the core genome. These genes are essential for basic functions and survival. The remaining 991 OGs are part of the accessory genome, which includes genes that may confer strain-specific traits. Notably, 277 of these accessory OGs are unique to only one strain, underscoring the genetic variation across different H. pylori strains.
- Restriction modification systems: The H. pylori genome contains 11 restriction modification systems, an unusually high number. These systems help defend against bacteriophages, or viruses that attack bacteria, by recognizing and cutting foreign DNA that could be harmful to the bacterium.
Geographical Distribution and Habitat of H. pylori Infection
Helicobacter pylori is a widespread bacterium that affects a large portion of the global population, although the exact prevalence remains difficult to determine, especially in developing countries.
Geographical Distribution
- Over 50% of the global population is infected with H. pylori.
- The true incidence rate of infection is unclear, particularly in developing countries, where accurate data is lacking.
- The bacterium’s distribution is widespread, suggesting that it can infect people across various regions, regardless of geographical boundaries.
Habitat
- H. pylori colonizes the gastric mucosa of both healthy individuals and those suffering from peptic ulcer diseases.
- It can survive in the acidic environment of the stomach by adhering to the mucosal lining, often without causing immediate symptoms.
- In individuals with peptic ulcers, the infection can lead to inflammation and damage, contributing to ulcer formation.
Culture and Biochemical Reactions of Helicobacter pylori
Helicobacter pylori is a bacteria that thrives in specific environmental conditions. To understand how it grows and the biochemical processes it undergoes, it’s important to look at both its culture requirements and biochemical characteristics.
Culture Conditions:
- Microaerophilic Growth: H. pylori needs reduced oxygen levels (around 5%) and elevated carbon dioxide levels (around 10%) to grow effectively.
- Temperature Range: The optimal temperature for growth is between 30°C and 37°C. It does not grow at 25°C and grows poorly at 42°C.
- Preferred Media: H. pylori grows best on freshly prepared chocolate agar or Skirrow’s campylobacter media. After incubation for 3-5 days, it forms circular, convex, and translucent colonies.
Biochemical Reactions:
- Urease Activity: H. pylori is highly proficient in producing the enzyme urease, which is about 100 times more active than that found in Proteus vulgaris. This enzyme plays a key role in neutralizing stomach acid, contributing to its ability to colonize the stomach lining.
- Catalase and Oxidase Positive: H. pylori tests positive for both catalase and oxidase. These enzymes help the bacteria survive in its hostile environment by breaking down reactive oxygen species.
- Sugar Metabolism: H. pylori does not ferment or oxidize sugars, making it biochemically inactive in this regard. However, it can metabolize amino acids via fermentative pathways. This ability helps it adapt to its environment, especially in the acidic stomach.
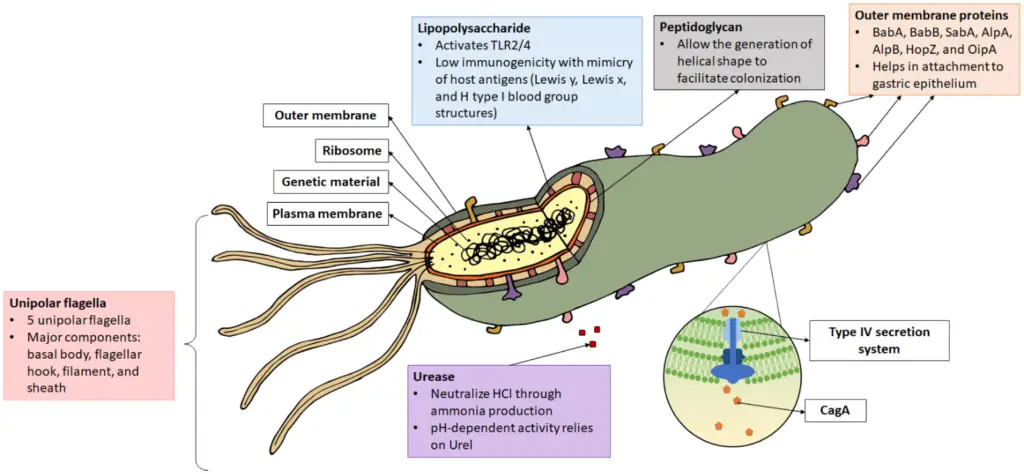
Virulence Factors of Helicobacter pylori
Helicobacter pylori employs a range of virulence factors that enable it to colonize the stomach, evade the immune system, and cause various gastric disorders. These factors contribute to its ability to persist in the harsh acidic environment of the stomach, and are integral to its pathogenicity.
Key Virulence Factors:
- Urease:
- The enzyme urease is central to H. pylori’s survival in the acidic stomach environment.
- It breaks down urea into ammonia, which neutralizes gastric acid near the bacterial cells, creating a more hospitable environment.
- Urease also plays a role in stimulating immune response, such as monocyte and neutrophil chemotaxis and the production of cytokines.
- Flagella:
- H. pylori’s flagella are crucial for motility and colonization.
- They allow the bacterium to penetrate the gastric mucous layer, where it can be protected from the stomach’s acidity, enhancing its ability to establish infection.
- Adhesins:
- These proteins enable H. pylori to attach firmly to the gastric mucosa.
- Types of adhesins include hemagglutinin, sialic acid-binding adhesins, and Lewis blood group adhesins, each contributing to the bacterium’s ability to bind to host cells and maintain its presence in the stomach.
- Enzymes:
- H. pylori produces various enzymes that assist in both survival and immune evasion.
- Mucinase and phospholipase break down the stomach’s protective mucus layer, allowing the bacteria to access the epithelial cells.
- Superoxide dismutase and catalase help the bacteria resist phagocytic killing by neutralizing harmful reactive oxygen species produced by immune cells.
- Heat Shock Protein (Hsp-B):
- This protein aids in the expression of urease, thereby facilitating H. pylori’s ability to survive in the acidic stomach environment.
- Acid Inhibitory Protein:
- This protein contributes to hypochlorhydria (a condition of reduced stomach acid) by blocking acid secretion from the parietal cells, further protecting H. pylori from stomach acidity.
- Cytotoxin:
- Cytotoxins produced by H. pylori cause vacuolation (the formation of vacuoles) in epithelial cells, contributing to tissue damage and inflammation.
- Vacuolating Toxin (VacA):
- Along with Cytotoxin-Associated Gene (CagA) protein, VacA causes vacuolation in host cells, further damaging gastric tissue and enhancing the pathogen’s ability to persist in the stomach lining.
Pathogenesis of H. pylori Infection
The pathogenesis of Helicobacter pylori infection involves a series of complex steps that allow the bacterium to colonize the stomach, evade the immune system, and cause damage to the gastric mucosa. This process is driven by several virulence factors that enable H. pylori to survive in the acidic environment of the stomach and interact with the gastric epithelium.
Key Stages in the Pathogenesis:
- Colonization of Gastric Mucosa:
- The infection begins when H. pylori colonizes the gastric mucosa.
- This colonization is supported by a variety of virulence factors, including acid-inhibitory proteins and urease.
- Acid-inhibitory proteins block acid production in the stomach, creating an environment conducive to the bacterium’s survival.
- Urease breaks down urea into ammonia, which neutralizes gastric acidity, further protecting the bacteria from the acidic conditions.
- Motility and Adhesion:
- Once H. pylori has neutralized the acid and created a safer environment, it uses its cork-screw motility to burrow through the gastric mucosa.
- This movement helps it reach the epithelial cells, where it can adhere firmly.
- Adhesion is mediated by several adhesin proteins, such as hemagglutinin, sialic acid-binding adhesin, and Lewis blood group adhesin, which allow the bacteria to bind tightly to the host’s cells.
- Immune Evasion:
- After adhesion, H. pylori must avoid immune system defenses.
- It uses enzymes like elastases and superoxide dismutase to protect itself from phagocytosis and intracellular killing by immune cells.
- These enzymes help H. pylori withstand the immune response and persist in the stomach.
- Tissue Damage:
- Damage to the epithelial cells occurs through a variety of mechanisms.
- Mucinases and phospholipases break down the protective mucus layer of the stomach.
- The bacteria also produce vacuolating toxins and the byproducts of urease, which further damage the host’s cells.
- This leads to inflammation and injury to the gastric lining.
- Long-term Effects:
- Chronic infection with H. pylori can cause atrophic and even metaplastic changes in the stomach lining, which are linked to the development of more serious conditions, such as gastric ulcers and gastric cancer.
What is CagA’s?
CagA plays a central role in the pathogenicity of Helicobacter pylori. Its presence and behavior can significantly influence the outcome of the infection. The mechanism of action behind CagA involves a series of complex steps that lead to cellular changes and increased disease severity.
- Surface exposure and variability: CagA is a hydrophilic, surface-exposed protein found in some H. pylori strains, but not all. The presence of the CagA-encoding gene, located on the cag pathogenicity island (cagPAI), helps classify strains into Cag+ (CagA-positive) or Cag− (CagA-negative) types.
- Prevalence in different populations: The prevalence of Cag+ H. pylori strains varies globally. In Egypt, Cag+ strains were found in about 33% of gastritis cases, 68.7% of peptic ulcer cases, and 50% of gastric cancer cases. Meanwhile, in Austria, 78% of peptic ulcer and 85% of gastric cancer isolates were Cag+ strains, highlighting the link between CagA presence and disease severity.
- Two types of CagA: There are two main variants of CagA, each with differing abilities to bind to cellular components. The East Asian CagA variant binds more strongly to the host protein SHP2 and is associated with higher virulence. In contrast, the Western CagA variant has a weaker binding affinity and is less pathogenic.
- Injection into host cells: CagA is injected into the host cell using the type IV secretion system (T4SS), which involves a complex mechanism. The T4SS consists of an outer membrane ring, an inner membrane ring, and a pilus that together transport CagA from the bacterial cell into the host cell cytoplasm.
- Phosphorylation and activation: Once inside the host cell, CagA undergoes phosphorylation at its EPIYA motif, a critical step for its activation. This phosphorylation is carried out by c-SRC and c-ABL kinases. The phosphorylated CagA then binds to the SH2 domain of host proteins, triggering several oncogenic signaling pathways.
- Impact on cell functions: The activation of these pathways leads to disruption of cell polarity, increased cell proliferation, and actin-cytoskeletal rearrangements. CagA also induces elongation of cells, disruption of tight and adherent junctions, proinflammatory responses, and suppression of apoptosis—all of which contribute to the pathogenesis of ulcers and cancer.
- Non-phosphorylated CagA: In some cases, CagA does not undergo phosphorylation but still induces cellular damage by interacting with host proteins like Grb2. This interaction leads to the loss of cell polarity, triggering mitogenic responses and proinflammatory signaling.
- Carcinogenesis: CagA also plays a key role in gastric carcinogenesis. It inhibits PAR1b-mediated BRCA1 phosphorylation, leading to DNA double-strand breaks and the stimulation of Hippo signaling, which contributes to genomic instability in cancer-prone cells. Additionally, the activation of YAP signaling by CagA promotes epithelial-mesenchymal transition, accelerating carcinogenesis and the spread of cancer in gastric epithelial cells.
CagA’s Mechanism of Action
CagA plays a central role in Helicobacter pylori‘s ability to cause disease, and its mechanism of action involves a precise interaction with the host cell.
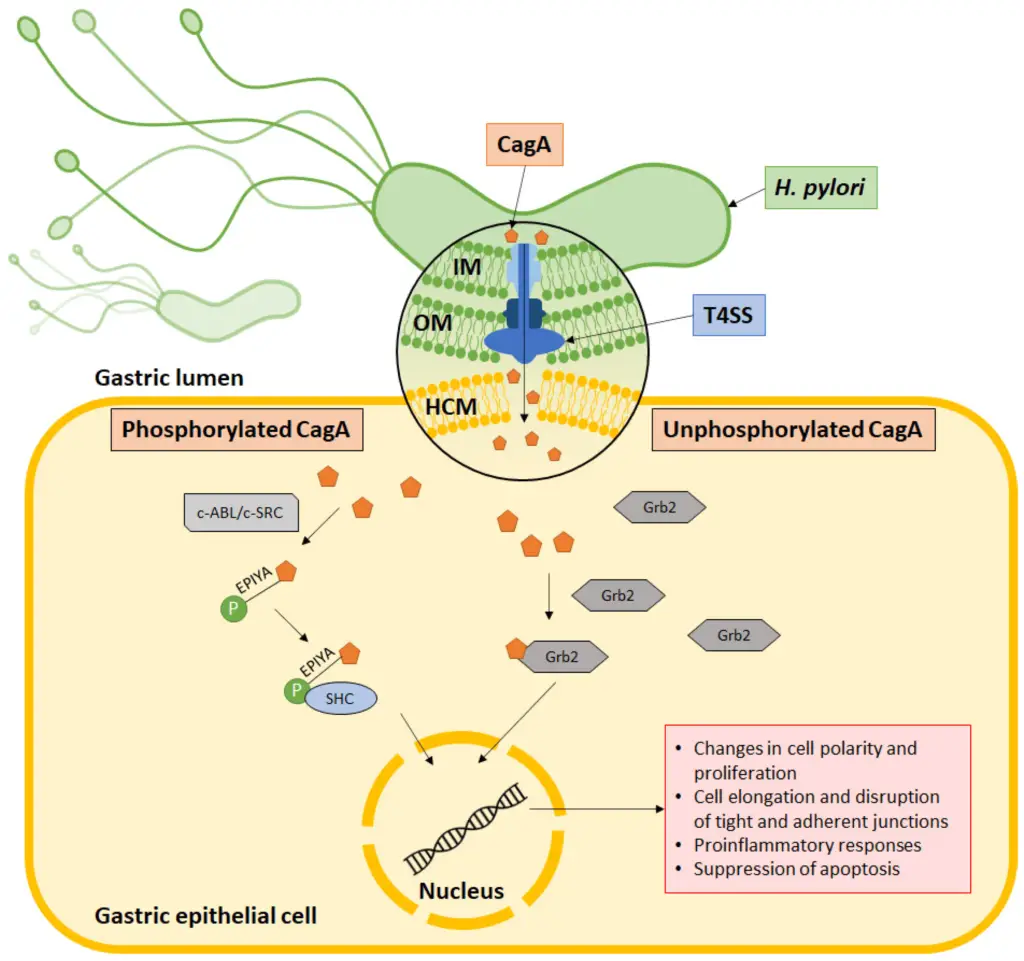
- Injection into host cells: CagA is delivered into the host cell via the type IV secretion system (T4SS). This complex system includes:
- An outer membrane ring in the bacterial outer membrane.
- An inner membrane ring in the bacterial inner membrane.
- A pilus that serves as the needle-like structure to inject CagA into the host cell membrane.
- Entry into the cytoplasm: Once the CagA protein is transported across the host cell membrane, it enters the cytoplasm, where it begins to exert its effects.
- Phosphorylation at the EPIYA motif: In some cases, CagA undergoes phosphorylation at a specific region known as the EPIYA motif. This modification is carried out by host cell kinases such as c-ABL or c-SRC. Phosphorylation is crucial for CagA’s interaction with other signaling molecules inside the host cell.
- Interaction with SHC domain: After phosphorylation, CagA associates with the SHC domain. This interaction leads to the induction of various pathologies, disrupting normal cell signaling.
- Alternative mechanism (Grb2 association): In other instances, CagA can also associate with the Grb2 protein without undergoing phosphorylation. This alternative mechanism still results in pathogenic effects within the host cell, highlighting the versatility of CagA in driving H. pylori-related diseases.
This complex and varied approach allows CagA to manipulate host cell signaling pathways, contributing to the formation of ulcers, inflammation, and other gastric issues associated with H. pylori infection.
Clinical Syndrome of H. pylori Infection
Helicobacter pylori infection is primarily linked to two major clinical syndromes: peptic ulcer disease and chronic atrophic gastritis. Both of these conditions are directly influenced by the presence of the bacterium in the gastric mucosa.
Peptic Ulcer Disease
- H. pylori is recognized as the primary cause of most gastric and duodenal ulcers.
- The infection leads to disruption of the gastric mucosal barrier, causing ulcers in the stomach and duodenum.
- The bacterium contributes to ulcer formation by increasing the production of gastric acid and causing inflammation of the gastric lining.
- Antibiotic treatment aimed at eradicating H. pylori from the stomach has proven effective in healing ulcers and dramatically reducing the likelihood of recurrence.
- Once the infection is cleared, the ulcers typically heal, and the recurrence rate drops significantly.
Chronic Atrophic Gastritis
- In the later stages of H. pylori infection, chronic atrophic gastritis may develop.
- This condition is characterized by the atrophy (thinning) of the gastric mucosa, leading to loss of normal function.
- It occurs as a result of long-term inflammation caused by the persistent presence of the bacterium.
- Chronic atrophic gastritis is a precursor to more severe conditions, including gastric cancer.
Reservoir, Source, and Transmission of H. pylori Infection
Helicobacter pylori primarily infects humans, making them the main source of transmission.
Reservoir and Source
- Humans are the primary reservoir of H. pylori infection.
- The prevalence of infection varies depending on geographical factors and race.
- About 60% of Hispanic individuals are affected.
- Around 54% of African Americans have the infection.
- Approximately 20% of White individuals carry the bacterium.
Transmission Routes
- The transmission of H. pylori is most commonly linked to poor hygiene, overcrowded living conditions, and poverty, which increase the risk of spread.
- The bacterium can spread through two main routes:
- Fecal-to-oral transmission: Infected stool contaminates food or water, which is then ingested.
- Oral-to-oral transmission: Stomach contents, including saliva, pass directly from one person to another through mouth-to-mouth contact.
Laboratory Diagnosis of H. pylori Infection
Diagnosing Helicobacter pylori infection involves various laboratory methods, each with distinct advantages and limitations. The right test depends on the clinical context and the patient’s history. Here’s a breakdown of the most common techniques used for diagnosing H. pylori.
1. Specimen Collection
- Stool Samples: Collected to detect antigens or bacteria.
- Gastric Biopsy Specimen: Obtained during endoscopy for direct examination or culture.
2. Microscopy
- Stains: Different staining methods, including Gram, Giemsa, Warthin Starry silver, and hematoxylin–eosin, are used to identify H. pylori under the microscope.
- These stains show the bacteria adhered to the gastric mucosa, helping confirm the presence of H. pylori.
- Specificity: Highly specific, ensuring reliable identification.
3. Culture
- H. pylori is cultured from clinical specimens on chocolate agar or Skirrow’s selective media.
- The incubation occurs at 35–37°C in a microaerophilic environment, with 5% oxygen, 10% carbon dioxide, and 85% nitrogen, for about 3–5 days.
- Colony Characteristics: The bacteria form large, convex, circular colonies, helping confirm their presence.
4. Bacterial Identification
- Once cultured, H. pylori is identified based on:
- Growth Characteristics: Appearance on selective media.
- Morphology: The shape of the bacteria.
- Biochemical Tests: Includes tests for oxidase, catalase, and urease (Box 36-2).
5. Serology
- ELISA: Enzyme-linked immunosorbent assay detects antibodies in the patient’s serum.
- Limitations: Although highly sensitive and specific, ELISA can’t differentiate between recent or past infections due to elevated antibody levels remaining long after infection.
6. Fecal Antigen Test
- An immunochromatographic test that detects H. pylori antigens in stool samples.
- Sensitivity: 94%
- Specificity: 98%
- It’s a highly accurate, non-invasive test.
7. Urease Test
- Procedure: A biopsy specimen is placed in a urease indicator medium, which contains urea and a pH indicator.
- Reaction: If H. pylori is present, it produces urease, which breaks down urea, changing the pH and color of the medium within minutes to 2 hours.
- Specificity: 100%
- Sensitivity: Ranges from 75% to 95%.
8. Rapid Urea Breath Test
- Method: Patients ingest a beverage containing a carbon-labeled urea. If H. pylori is present, it breaks down the urea, releasing labeled carbon that can be detected in the breath.
- Accuracy: Selective for H. pylori, it’s a reliable test for active infection.
- Potential Issues: False positives can occur due to:
- Infection with non-urease-producing coccoid forms of H. pylori.
- Use of antibiotics like bismuth or histamine-2 blockers, which can interfere with the test.
Treatment of H. pylori Infection
H. pylori infections are commonly treated with a combination of antibiotics and other drugs. The key to treatment is using the right mix of medications to ensure effectiveness.
- Antibiotics alone are not enough. H. pylori is sensitive to several antibiotics and bismuth salts, but using a single antibiotic by itself won’t get the job done.
- Triple therapy is the standard approach. This involves a combination of:
- Bismuth salts
- A proton pump inhibitor (PPI), such as omeprazole
- One or more antibiotics. Common choices include ampicillin, metronidazole, clarithromycin, and tetracycline.
- Treatment duration matters. Triple therapy is typically given for either 10 or 14 days, depending on the case.
- Resistance is a concern. Some H. pylori strains are becoming resistant to macrolide antibiotics, including clarithromycin. This is especially seen in children, where strains resistant to clarithromycin have been documented.
- Bismuth compounds help. Bismuth salts are commonly added because they have properties that help kill the bacteria and protect the stomach lining, improving the effectiveness of other treatments.
- Always check for resistance. The emergence of antibiotic-resistant strains makes it essential to test for resistance before starting treatment, particularly in cases of recurring infections.
Other Helicobacter Species
While Helicobacter pylori often takes the spotlight, several other Helicobacter species are worth noting. Some of these species are primarily studied in animals, while others have been linked to human infections under specific circumstances.
- H. fennelliae and H. cinaedi: These species were once classified under the Campylobacter genus but were later reclassified to Helicobacter. They have been found in homosexual men with concurrent HIV and tuberculosis infections. These individuals typically suffer from conditions like proctitis, protocolitis, or enteritis.
- Helicobacter-like organisms: Newer species, like H. mustelae and H. hepaticus, have been discovered in animals. H. mustelae was first identified in ferrets, and H. hepaticus was found in Syrian hamsters using specific PCR tests.
- No human infections reported: Despite being related to H. pylori, these Helicobacter-like organisms do not cause infections in humans. Instead, they are often used as animal models for studying H. pylori infections, providing insights into treatment and diagnosis.
- Biochemical differences: While all these species share similarities, they differ in key biochemical properties. For example, growth temperatures, urease activity, and nitrate reduction can vary among species. These differences are important for both identification and understanding the ecological niches of each organism.
- Animal models for research: The animal species that host these Helicobacter strains offer valuable insights into the bacterial behavior and mechanisms that could apply to human H. pylori infections. Studies on these species help researchers understand the broader family of Helicobacter organisms.
- Parikh NS, Ahlawat R. Helicobacter Pylori. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534233/
- Cheok, Y. Y., Lee, C. Y. Q., Cheong, H. C., Vadivelu, J., Looi, C. Y., Abdullah, S., & Wong, W. F. (2021). An Overview of Helicobacter pylori Survival Tactics in the Hostile Human Stomach Environment. Microorganisms, 9(12), 2502. https://doi.org/10.3390/microorganisms9122502
- https://emedicine.medscape.com/article/176938-overview
- https://www.mayoclinic.org/diseases-conditions/h-pylori/diagnosis-treatment/drc-20356177
- https://www.mayoclinic.org/diseases-conditions/h-pylori/symptoms-causes/syc-20356171
- https://www.webmd.com/digestive-disorders/h-pylori-helicobacter-pylori
- https://www.hopkinsmedicine.org/health/conditions-and-diseases/helicobacter-pylori
- https://my.clevelandclinic.org/health/diseases/21463-h-pylori-infection
- https://www.healthline.com/health/helicobacter-pylori
- https://medlineplus.gov/helicobacterpyloriinfections.html
- https://www.uptodate.com/contents/helicobacter-pylori-infection-and-treatment-beyond-the-basics/print
- https://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/h-pylori-fact-sheet
- https://en.wikipedia.org/wiki/Helicobacter_pylori
- https://www.healthdirect.gov.au/helicobacter-pylori
- https://patient.info/digestive-health/dyspepsia-indigestion/helicobacter-pylori